Uroleucon ( Uroleucon ) lebanonense, Kanturski & Barjadze, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4853.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:B070AFD0-5474-41F2-8A92-5E6F41584D08 |
|
DOI |
https://doi.org/10.5281/zenodo.4519759 |
|
persistent identifier |
https://treatment.plazi.org/id/E82CA06B-35E6-4333-AECD-F8B310783FE3 |
|
taxon LSID |
lsid:zoobank.org:act:E82CA06B-35E6-4333-AECD-F8B310783FE3 |
|
treatment provided by |
Plazi |
|
scientific name |
Uroleucon ( Uroleucon ) lebanonense |
| status |
sp. nov. |
Uroleucon ( Uroleucon) lebanonense sp. nov.
urn:lsid:zoobank.org:act:
( Figs 1 –5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 , Table 1)
Type material: HOLOTYPE. Apterous viviparous female marked with black circle and “H”, LEBANON, 13 km S. E. of Jazzine, 30.v.1973, on Tragopogon phaenopappus (= T. coloratus as it was written on the original label), D. Hille Ris Lambers leg., NHMUK 010120592, BMNH.
PARATYPES. 3 apterous viviparous females, NHMUK 010120592, BMNH ; 2 apterous viviparous females, NHMUK 010120564, BMNH; 3 apterous viviparous females, NHMUK 010120551; 2 apterous viviparous females, NHMUK 010120574, BMNH; 3 apterous viviparous females, NHMUK 010120552, BMNH; 2 apterous viviparous females, NHMUK 010120591, BMNH; 3 apterous viviparous females, NHMUK 010120571, BMNH; 4 apterous viviparous females, NHMUK 010120584, BMNH; 4 apterous viviparous females, NHMUK 010120563, BMNH; 3 apterous viviparous females, NHMUK 010120569, BMNH; 4 apterous viviparous females, NHMUK 010120572, BMNH; 3 apterous viviparous females, NHMUK 010120570, BMNH; 3 apterous viviparous, 1 alate viviparous female, NHMUK 010120583, DZUS, 3 apterous viviparous, females, NHMUK 010120553, IZISU, other data as in holotype.
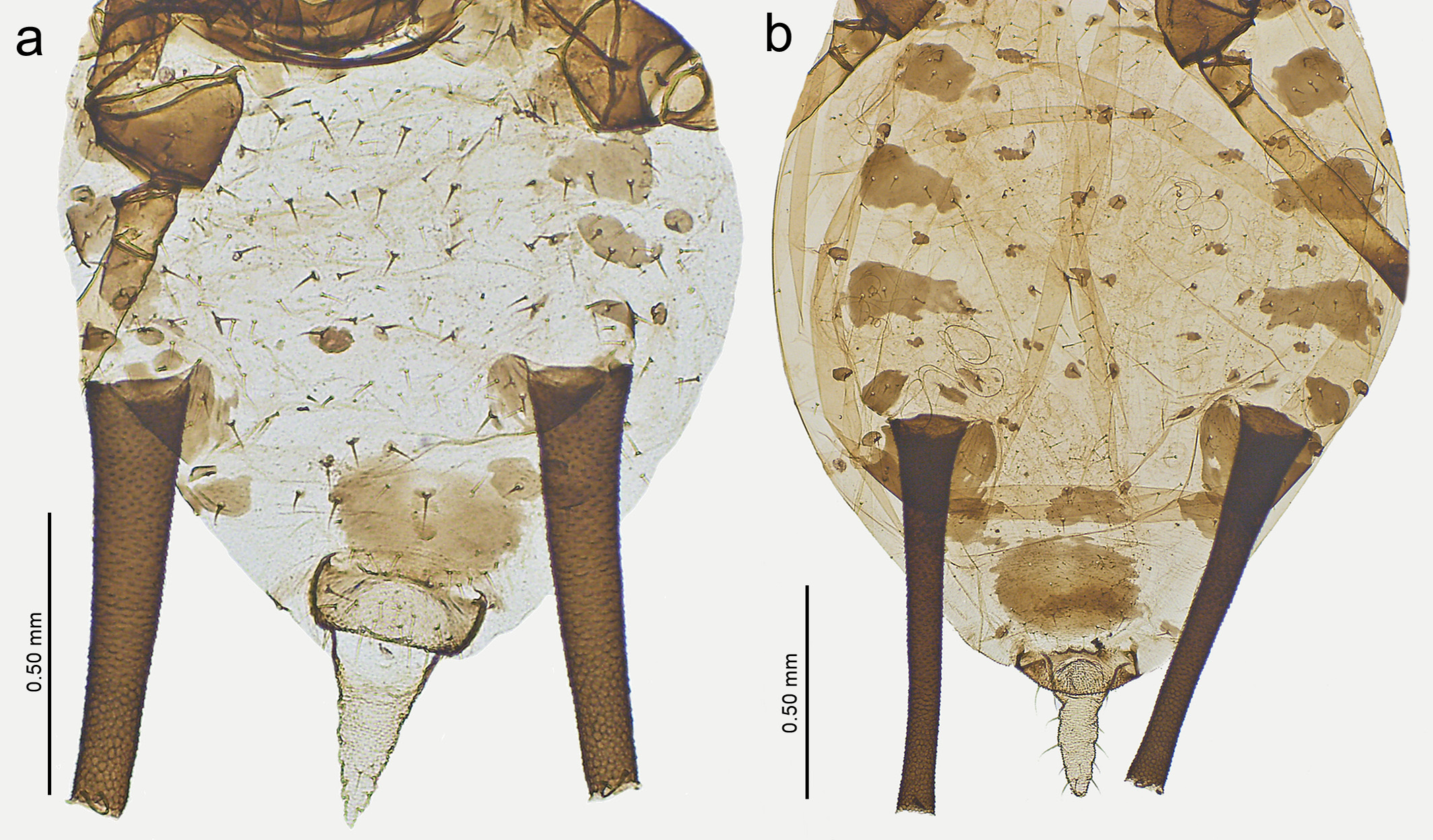
Description. Apterous viviparous female (n=43). Colour in life: unknown. Pigmentation on slide: head sclerotized, brown; ANT I–II dark brown; ANT III brown with pale bases; ANT IV pale with brown apices or rarely basal halves pale and apical halves brown; ANT V pale with brown apices or completely brown; ANT VI brown; pronotum and mesonotum usually sclerotized, brown; coxae dark brown; trochanters light brown; femora yellow with light brown or brown distal halves; tibiae with yellow middle section and brown bases and apices; tarsi brown; abdomen yellow with brown sclerites and scleroites; SIPH completely brown, cauda pale ( Fig. 1a View FIGURE 1 ). HW 0.19–0.21 × ANT. Head with thick, rigid setae with slightly blunt or narrowly capitate apices, 0.030 –0.055 mm long. ANT tubercles each with 2–3 setae on internal angles, 0.030 –0.050 mm long. ANT 0.86–1.04 × BL. ANT III with 45–59 rounded, different-sized secondary rhinaria, with sclerotized rims ( Fig. 2a View FIGURE 2 ), ANT IV longer than ANT V. PT 5.52– 6.43 × BASE. Other antennal ratios: VI:III 0.98–1.14, V:III 0.44–0.48, IV:III 0.51–0.68, PT:III 0.83–0.98, PT:IV 1.32–1.36, PT:V 1.76–2.05. ANT chaetotaxy: ANT bearing thick, rigid setae with slightly blunt or narrow capitate apices. ANT III setae 0.015 –0.040 mm long, LS ANT III 0.74–0.88 × BD III. ANT I with 6–10, ANT II with 4–6, ANT III with 15–24, ANT IV with 9–13, ANT V with 6–8 setae. ANT VI with 2–3 basal, 4 apical and subapical and 3–7 setae along the PT. Rostrum extending back beyond hind coxae. URS 0.24–0.26 × ANT III, 0.23–0.25 × ANT VI, 0.27–0.30 × PT, 1.68–1.77 × BASE and 1.17–1.33 × HT II, with 7–10 short, fine, pointed accessory setae ( Fig. 2c View FIGURE 2 ). Mesosternal furca fused, wide, without stem. III FEMORA bearing short, thick, rigid setae with narrow capitate or slightly blunt setae, 0.015 –0.040 mm long. III TIBIAE bearing thick, rigid setae with mostly slightly pointed or blunt, 0.010 –0.050 mm long. HT I with 3:3:3 ventral setae, HT II 0.19–0.21 × ANT III, 0.17–0.21 × ANT VI, 0.20–0.24 × PT and 1.28–1.44 × BASE. SIPH tubular, slightly tapering, straight, with distinct zone of subapical reticulation and without flange ( Fig. 2e View FIGURE 2 ). The reticulated zone 0.36–0.39 × SIPH. SIPH 1.58–1.93 × cauda, 0.20–0.28 × BL, and 0.80–0.91 × ANT III. Abdomen membranous, without marginal tubercles, with medium in length, thick, rigid setae with narrowly capitate or blunt apices, 0.035 –0.055 mm long on ABD TERG I–V and 0.045 –0.065 mm long on ABD TERG VI–VIII which arise from clearly visible, rounded and irregular scleroites in spinal, dorsal and marginal areas ( Fig. 3a, c View FIGURE 3 ). SIPH surrounded by always fragmented antesiphuncular and well–developed postsiphuncular sclerites. Genital plate with two anterior setae that are longer than the others, 10–16 posterior setae. Cauda tapering, 1.65–2.05 × its width at base and 0.12–0.15 × BL, with 12–18 fine setae of two lengths ( Fig. 2g View FIGURE 2 ).
Description. Alate viviparous female (n=1). Colour in life: unknown. Pigmentation on slide: head and thorax sclerotized, brown; ANT brown except basal part of ANT III and ANT IV and V which have only brown apical parts; coxae dark brown; trochanters light brown; femora with yellow with brown distal halves; tibiae with yellow middle section and brown apices (knee area) and bases; tarsi brown; SIPH brown; cauda pale ( Fig. 1c View FIGURE 1 ). HW 0.18–0.19 × ANT. Head with thick and rigid setae with blunt or narrow capitate apices, 0.020 –0.035 mm long. ANT tubercles each with 3 setae on internal angles. ANT 1.14–1.18 × BL. ANT III with 60–66 rounded, different sized, secondary rhinaria with sclerotized rims distributed over most of length ( Fig. 4a View FIGURE 4 ), ANT IV longer than ANT V. PT 4.60–6.50 × BASE. Other antennal ratios: VI:III 1.05–1.06, V:III 0.41–0.43, IV:III 0.56–0.58, PT:III 0.86–0.92, PT:IV 1.53– 1.56, PT:V 2.09–2.11. ANT bearing short thick and rigid setae with blunt or narrowly capitate apices. ANT III setae 0.010 –0.025 mm long, LS ANT III 0.55–0.62 × BD III. ANT I with 9, ANT II with 5, ANT III with 22–23, ANT IV with 11–12, ANT V with 8 setae. ANT VI with 2–3 basal, 4 apical and 4–5 setae along the PT. Rostrum reaching metasternum. URS 0.25 × ANT III, 0.23–0.24 × ANT VI, 0.27–0.28 × PT, 1.33–1.81 × BASE and 1.25–1.29 × HT II, with 9 fine and pointed accessory setae ( Fig. 4c View FIGURE 4 ). III FEMORA bearing short, thick, rigid setae with narrowly capitate or blunt, 0.010 –0.030 mm long. III TIBIAE bearing thick, rigid setae with mostly slightly pointed or blunt apices, 0.015 –0.035 mm long. HT I with 3:3:3 ventral setae, HT II 0.19–0.20 × ANT III, 0.18–0.19 × ANT VI, 0.22 × PT and 1.03–1.45 × BASE. SIPH tubular, slightly tapering, straight, with distinct zone of subapical reticulation and without flange ( Fig. 4e View FIGURE 4 ). The reticulated zone 0.29–0.32 × SIPH. SIPH 2.21 × cauda, 0.26 × BL, and 0.77–0.79 × ANT III. Abdomen membranous without marginal tubercles, with short thick and rigid setae with narrowly capitate or blunt apices, 0.034 –0.045 mm long on ABD TERG I–V and 0.035 –0.055 mm long on ABD TERG VI–VIII. ABD VIII with 4 setae. Scleroites at setal bases very few, only on ABD TERG V–VII. SIPH surrounded by ante– and postsiphuncular sclerites. Marginal sclerotic plates bigger on ABD TERG I–II than those on ABD TERG III. Genital plate with two anterior setae which are longer than the others, 12 posterior setae. Cauda tapering, without constriction, 1.47 × its width at base and 0.12 × BL, with 14 fine setae of two lengths ( Fig. 4g View FIGURE 4 ).
Differential diagnosis. There are the seven Palaearctic species currently placed in Uroleucon with 3:3:3 setae on the first tarsal segments: Uroleucon bielawskii , U. dubium Holman, 1975 , U. kashmiricum ( Verma, 1966) , U. mongolicum , U. mulgedii , U. pilosellae ( Börner, 1933) , and U. telekiae ( Holman, 1965) ( Nevsky 1928; Szelegiewicz 1962, 1982; Holman 1965, 1975, 1991; Verma 1966).
Apterous viviparous females of Uroleucon ( Uroleucon) lebanonense differ from the same morphs of U. bielawskii , U. dubium and U. mulgedii because they are classified into different subgenera according to the taxonomic criteria used by the authors ( Szelegiewicz 1962, 1982; Holman 1975; Blackman & Eastop 2020).Apterae of U. lebanonense differ from the same morphs of U. kashmiricum by ( 1) number of secondary rhinaria on ANT III: 45–59 secondary rhinaria in the new species, while 10–30 secondary rhinaria are present in U. kashmiricum , ( 2) number of setae on ABD TERG VIII: 4–7 in the new species, while only 2 in U. kashmiricum , ( 3) SIPH/cauda ratio: 1.58–1.93 in the new species, while 1.30–1.33 in U. kashmiricum , ( 4) presence/absence of antesiphuncular sclerites: fragmented or vestigial sclerites present in the new species, while they are absent in U. kashmiricum ( Verma 1966; Blackman & Eastop 2020). From U. pilosellae , the apterous morphs of U. lebanonense may be distinguished by ( 1) number of secondary rhinaria on ANT III: 45–59 secondary rhinaria in the new species, while 15–28 secondary rhinaria in U. pilosellae , ( 2) fragmented or vestigial sclerites are present in the new species, while large and solid sclerites are present in U. pilosellae ( Heie 1995; Blackman 2010; Blackman & Eastop 2020). Apterous viviparous females of U. lebanonense differ from the same morphs of U. telekiae by ( 1) number of secondary rhinaria on ANT III: 45–59 secondary rhinaria in the new species, while 6–25 secondary rhinaria in U. telekiae , ( 2) URS/HT II ratio: 1.17–1.33 in the new species, while 1.50–1.80 in U. telekiae , ( 3) SIPH/BL ratio: 0.20–0.28 in the new species, while 0.32–0.38 in U. telekiae , ( 4) number of setae on cauda: 12–18 in the new species, while 20–26 in U. telekiae ( Holman 1965; Heie 1995; Blackman & Eastop 2020).
Among all known Palaearctic species of the subgenus Uroleucon with 3:3:3 setae on HT I, the new species is most similar to U. mongolicum , with a similar number of setae on ABD TERG VIII ( 4–7 in the new species and 3–5 in U. mongolicum ). However, apterous viviparous females of U. lebanonense are characterized by several differences in contrast to U. mongolicum :
• Mesosternal furca without stem (furca with robust stem in U. mongolicum );
• Cauda with 12–18 setae ( Fig. 2g View FIGURE 2 ) (cauda with 7–11 setae in U. mongolicum , ( Fig. 2h View FIGURE 2 ));
• SIPH tapering gradually from base to apex ( Fig. 2e View FIGURE 2 ) (SIPH with subapical constriction and slightly flared apex ( Fig. 2f View FIGURE 2 ));
• Lower ratio of SIPH/cauda—1.58–1.93 ( 1.80-2.60 in U. mongolicum respectively);
• Secondary rhinaria with sclerotized rims (fig. 2a) (secondary rhinaria without sclerotized rims in U. mongolicum ( Fig. 2b View FIGURE 2 ));
• Dorsum of abdomen smooth without reticulation ( Fig. 3c View FIGURE 3 ) (dorsum of abdomen with well–developed reticulation in U. mongolicum ( Fig. 3d View FIGURE 3 ));
• Higher ratio of URS/ANT III—0.24–0.26 and URS/ANT VI—0.23–0.25 (0.16–0.21 and 0.16–0.19 in U. mongolicum respectively).
Alate viviparous females of the new species can be distinguished from the same morph of U. mongolicum by:
• SIPH tapering gradually from base to apex ( Fig. 4e View FIGURE 4 ) (SIPH with distinctly constricted reticulation area in U. mongolicum ( Fig. 4f View FIGURE 4 ));
• Secondary rhinaria with sclerotized rims ( Fig. 4a View FIGURE 4 ) (secondary rhinaria without sclerotized rims in U. mongolicum ( Fig. 4b View FIGURE 4 ));
• Cauda elongate triangular without constriction near base, with 14 setae ( Fig. 4g View FIGURE 4 ) (cauda finger–like constricted near base, with 7–11 setae in U. mongolicum ( Fig. 4h View FIGURE 4 );
• Marginal sclerites on ABD TERG IV smaller than those on ABD TERG III ( Figs 1c View FIGURE 1 , 5a View FIGURE 5 ) (marginal sclerites on ABD TERG IV as big as or larger than those on ABD TERG III in U. mongolicum ( Figs 1d View FIGURE 1 , 5b View FIGURE 5 ));
• Higher ratio of ANT IV/ANT V—1.35–1.36, URS/ANT VI—0.23–0.24 and HT II/ANT III—0.19–0.20 (1.22– 1.25, 0.15–0.19 and 0.13–0.14 in U. mongolicum respectively).
Etymology. Name of the new species is derived from Lebanon, where it has been collected.
Biology and distribution. So far Uroleucon lebanonense is known only from its type locality in the vicinity of Jazzine in Lebanon. Other morphs and life cycle of the new species are unknown. Hille Ris Lambers put the name Tragopogon coloratus as the plant from which the new species has been collected. However T. coloratus is known to be native to Iran, Transcaucasus (including Armenia, Azerbaijan and Georgia) and Turkey ( Kilian et al. 2009). In Lebanon there are records for T. buphthalmoides , T. coelesyriacus , T. porrifolius , and T. pterocarpus ( Kilian et al. 2009) so there may have been a misidentification of the reported host plant.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |