Etheophanus Broun, 1893
|
publication ID |
https://doi.org/10.11646/zootaxa.4543.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:58142B27-5E25-46EE-A1B0-71BB31DFB3E2 |
|
DOI |
https://doi.org/10.5281/zenodo.5944053 |
|
persistent identifier |
https://treatment.plazi.org/id/03DEC54A-FFE9-C91B-FBA8-4081FC72FBFD |
|
treatment provided by |
Plazi |
|
scientific name |
Etheophanus Broun, 1893 |
| status |
|
Etheophanus Broun, 1893 View in CoL
( Figs 1–15 View FIGURES 1 View FIGURES 2 View FIGURES 3 View FIGURES 4 View FIGURES 5 View FIGURES 6 View FIGURES 7 View FIGURES 8 View FIGURES 9 View FIGURES 10 View FIGURES 11 View FIGURES 12 View FIGURES 13 View FIGURES 14 View FIGURES 15 )
Etheophanus Broun, 1893: 1232 View in CoL . Type species: Etheophanus pinguis Broun, 1893 View in CoL , by monotypy.
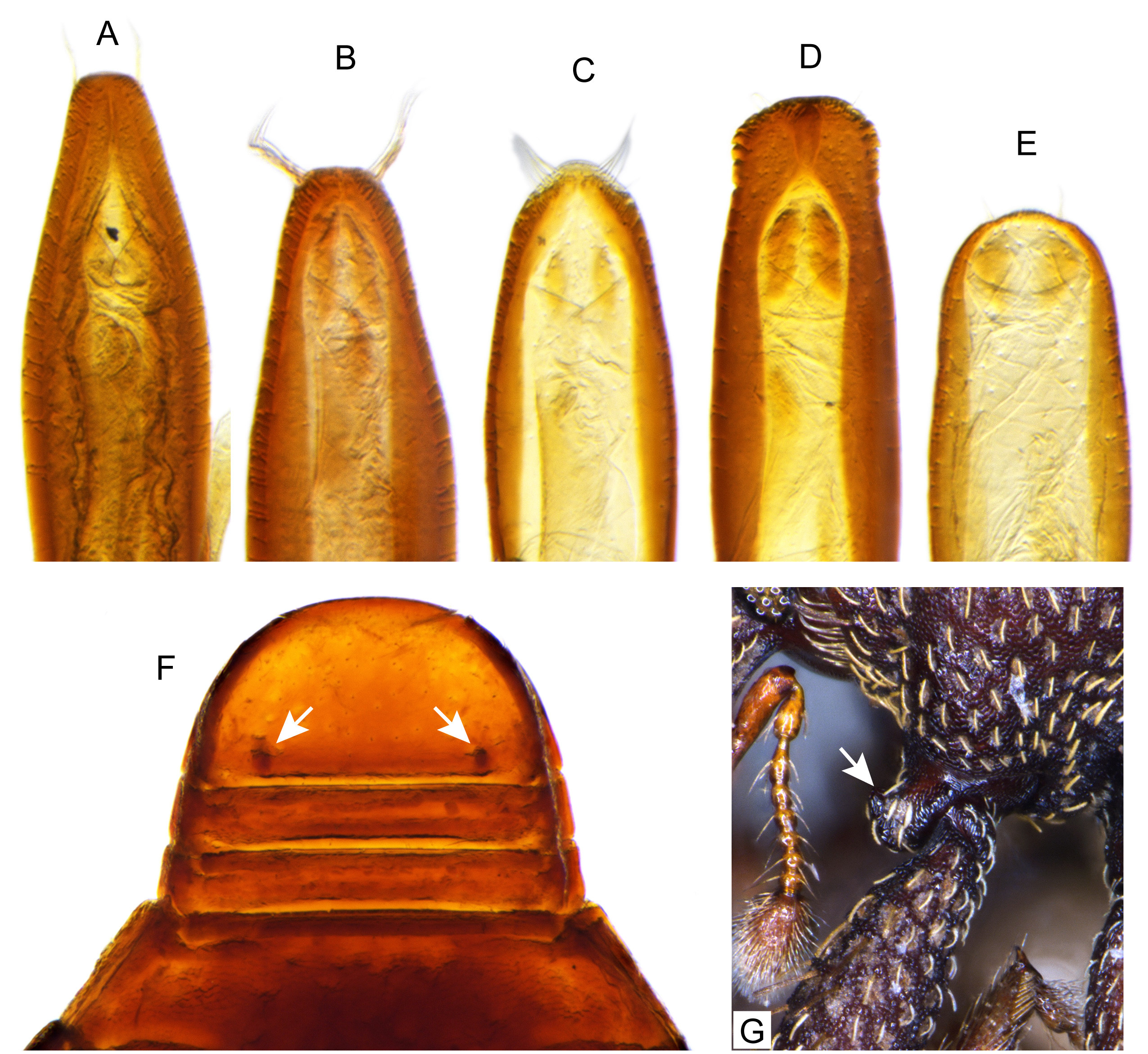
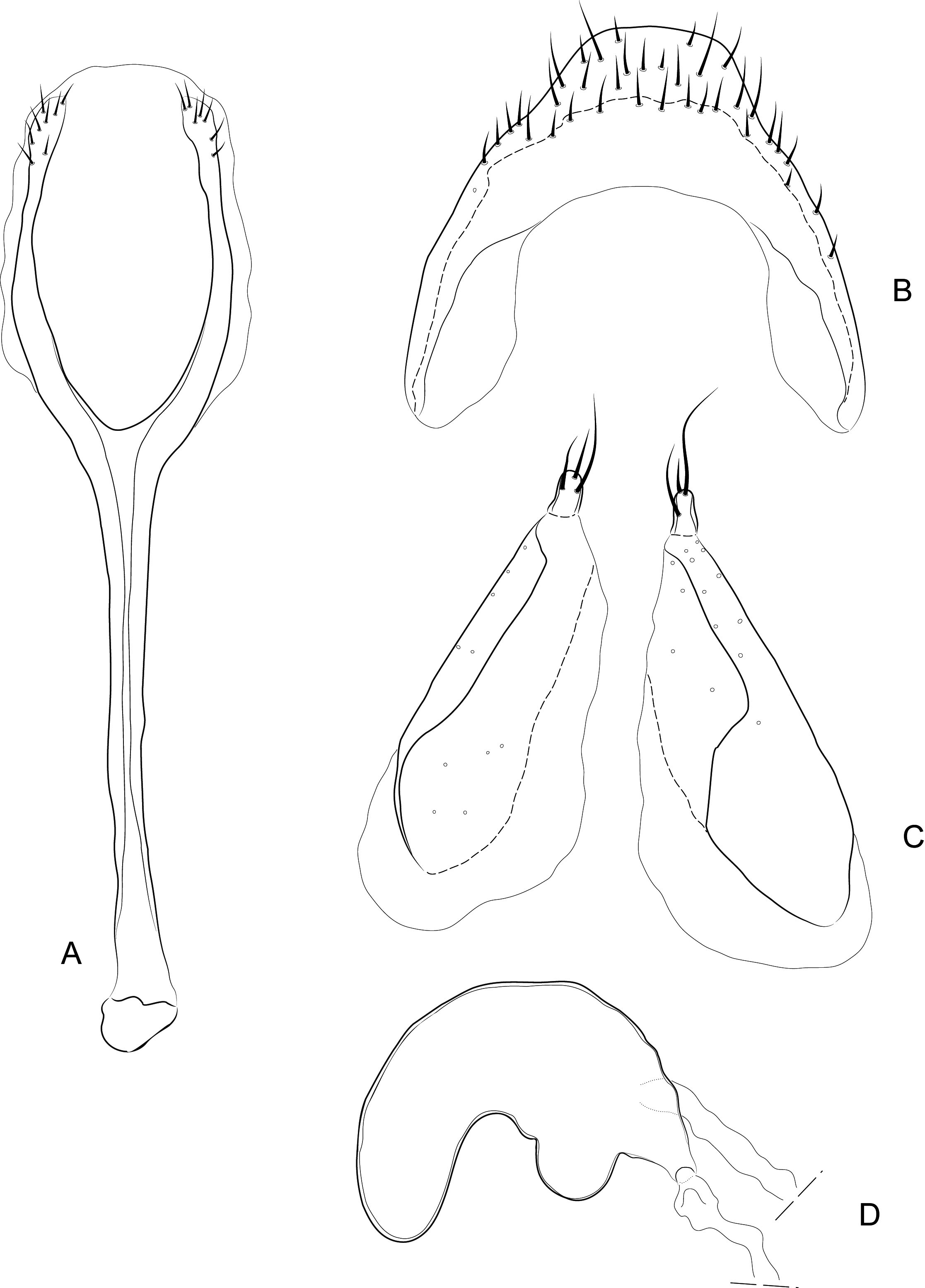
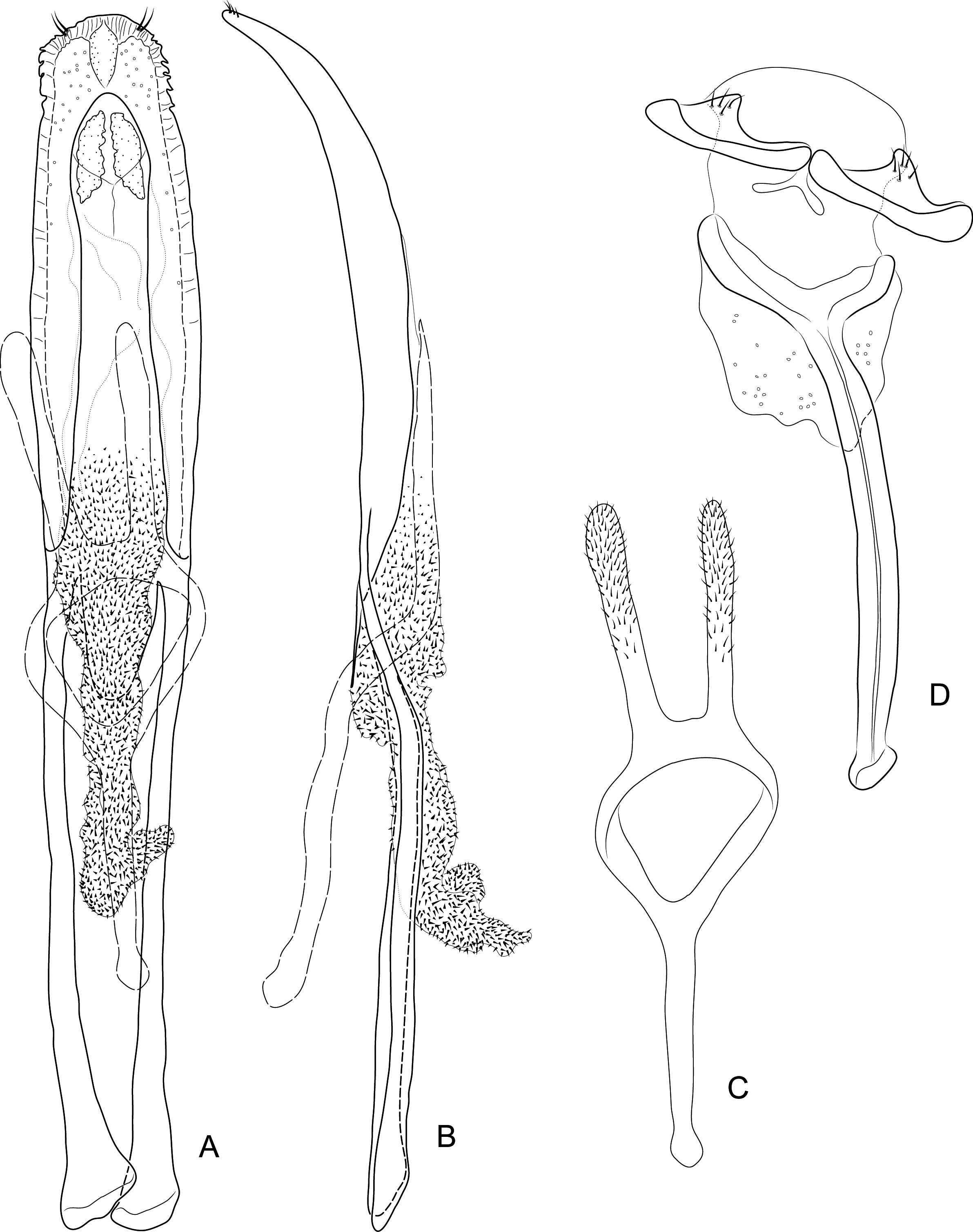
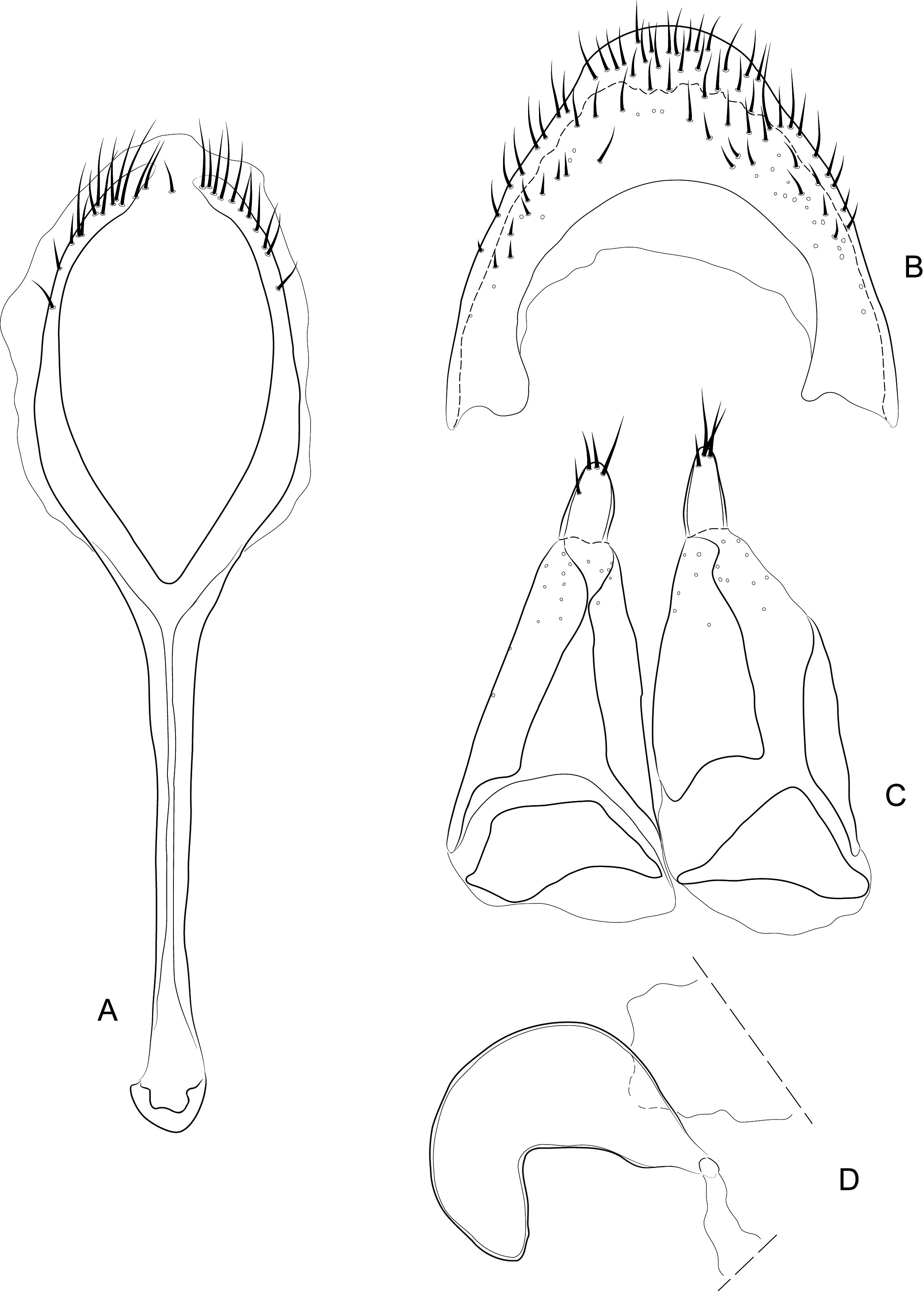
Diagnosis. Body compact and often covered in encrustation. Colour light to dark red-brown, often with black pigments at the humeral, subapical and central areas of the elytra. Vestiture of thick narrow setae, that are white, golden or black, biseriate consisting of decumbent primary and scattered erect setae that often are widened or frayed apically. Mouthparts: labium ( Fig. 4A View FIGURES 4 ) with 3-segmented palpus; basal segment with one to two dorsal setae, middle segment lacking setae or with one dorsal seta, apical segment lacking setae, with 6 apical sensilla; prementum with two dorsal setae (one at each dorsolateral corner); ligula lightly sclerotized, acute; mandibles weakly falcate; left mandible ( Fig. 4C View FIGURES 4 ) with three incisors and short molar region, middle incisor larger, inner incisor small, and one seta along outer dorsal margin; maxilla ( Fig. 4B View FIGURES 4 ) with 3-segmented palpus, basal segment with one seta near outer apical margin, middle and apical segments lacking setae; palpiger with two dorsal setae; galeolacinial complex with large, paddle-shaped setae along mesal margin. Rostrum with lateral carinae, often conjoined before eyes with an internal area subglabrous and not highly polished, length about as long as prothorax; antennae inserted apically, about 1/5 the total distance of rostrum from eye. Pronotum narrower than combined width of elytra with sides more or less parallel-sided and not strongly convex; anterior not strongly constricted and extended apically forming a hood; pronotum and elytra at different levels. Proventrite lacking groove for retraction of rostrum. Scutellar shield absent. Elytra with five deep to weakly impressed apunctate striae, apex rounded to attenuate. Mesoventrite lacking a rostral groove. Sclerolepidia present and squamiform. Total length of abdomen about 1/3 longer than meso- and metaventrites combined. Abdominal ventrite 5 of male with a pair of setae arising from a cluster of circular pores. Tibiae bearing a single apical spine.
Remarks. Placement of Etheophanus within the Molytinae is supported by the form of the pharyngeal plate ( Fig. 4D View FIGURES 4 ; Davis 2017) whereby the anterior arms are long and narrow, the plate is nearly divided medially by a long notch (and only narrowly connected posteriorly), and the posterior region of the plate possesses only a few small holes. Two additional characters supporting the molytine placement is the presence of a small spiculum relictum in the male ( Figs. 6D View FIGURES 6 ) and a pair of small internal apodemes on the anterolateral corners of the 5 th abdominal ventrite of the female ( Fig. 5F View FIGURES 5 ). Although these latter two characters are not restricted to molytines and are found elsewhere (e.g., the spiculum relictum in Nemonychidae , Belidae , some Attelabidae , Brentidae , Caridae , and several curculionid subfamilies, and the internal abdominal apodemes at least in the curculionid subfamilies Hyperinae , Cyclominae , and Entiminae, S. Brown, pers. comm.), homoplasious characters are inherent in phylogenetics. After all, it is the distinct suite of traits that defines a taxon, not any particular trait in isolation. The spiculum relictum does appear in several curculionoid lineages and, as expected of any feature that is gained or lost several times through evolution, it displays slight differences in the various lineages. In molytines, the apex is narrow and strongly bifid. Lyal (2014) proposed that Etheophanus was a member of monophyletic group containing Exeiratus Broun , Inososgenes Broun , Paedaretus Pascoe , and Phronira Broun supported by the presence of sclerolepidia, weak postocular lobes, an emarginate prosternal anterior margin, and an indication of a prosternal canal. These genera are mostly New Zealand endemics apart from Exeiratus which is shared with Australia and COI and 28S data (Leschen et al., in prep.) corroborate this hypothesis of monophyly.
There are five species of Etheophanus , one described here, which have a wide range of colour and setal patterning ( Figs. 1 View FIGURES 1 & 2 View FIGURES 2 ), characters that were used to name and recognise the species by Broun. However, we have found that there are few consistent size, colour and vestiture characters among the species. Craw (1988) scored characters for a cladistic analysis, some characters of which we could not use effectively include shape (the elytra subcordate versus subrounded, level of pronotal and elytral surfaces) and six male genitalic characters, and only two we have confirmed here. The other aedeagal characters may have been either scored in error or were not examined with a stereomicroscope because all species have an ostiolar canal present with valves (which are generally membranous and weakly pigmented in most specimens) and the sclerite of the internal sac appears not to be a sclerite, but rather a broad region of spines, with an overall shape that does not seem to correspond to the character states in Craw’s data matrix. The area consists of small, weakly pigmented elongate spines in E. optandus , small, weakly pigmented and dense hair like processes in E. pinguis and E. striatus , and small, dense toothlike processes in E. nitidellus . Nonetheless, differences in the size, pigmentation, and spatial orientation of these endophallic spines are indeed subtle.
Other than colour, vestiture, and body shape, there are few additional external characters reliable for separating the taxa that are included in the key and diagnoses for the species. Punctation, as used by Craw (1988) is a useful character. Two autapomorphies are the unusual process on the procoxae of E. kuscheli ( Figs. 2A View FIGURES 2 , 3A View FIGURES 3 , 5G View FIGURES 5 ) and the absence of pronotal vibrissae in E. striatus (presence of these are easily seen behind the eye and on the lower anterior margin of pronotum in Figs. 2A View FIGURES 2 , 3A View FIGURES 3 ). Indicated by our genetic study, there may be a complex of species among populations currently considered as E. nitidellus and E. optandus , and more dissections are required to determine species status and variation in the genitalia. Further collecting is needed to evaluate exact population limits because of gaps in genetic sampling along the entire range of the species; particularly, the nitidellus / optandus complex which extends from Westland south to Stewart Island and E. striatus which is present in eastern portions of the South Island and as far south as Dunedin. We fully describe one new species and provide diagnostic descriptions for the remaining species.
The biology of the genus is poorly known, beyond that all species are associated with leaf litter. The lack of collections of specimens in Lynfield, Auckland ( Kuschel 1990) may indicate special habitat or food plant requirements, or that they do not thrive well in urban environments.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Etheophanus Broun, 1893
| Davis, Steven R., Brav-Cubitt, Talia, Buckley, Thomas R. & Leschen, Richard A. B. 2019 |
Etheophanus Broun, 1893 : 1232
| Broun, T. 1893: 1232 |