Termitaria hexasporodochia Wilson, Emam, Davis, Barden, Hall, Ware., 2023
|
publication ID |
https://doi.org/10.11646/phytotaxa.591.2.3 |
|
DOI |
https://doi.org/10.5281/zenodo.7797490 |
|
persistent identifier |
https://treatment.plazi.org/id/03E06130-F64E-FFBB-FF77-0F9AFC961ED9 |
|
treatment provided by |
Plazi |
|
scientific name |
Termitaria hexasporodochia Wilson, Emam, Davis, Barden, Hall, Ware. |
| status |
sp. nov. |
Species: Termitaria hexasporodochia Wilson, Emam, Davis, Barden, Hall, Ware. sp. nov.
Mycobank ID: MB832822
Newark Museum Herbarium, Newark, NJ (NEMU)
American Museum of Natural History, NYC, NY (AMNH)
Type Specimens
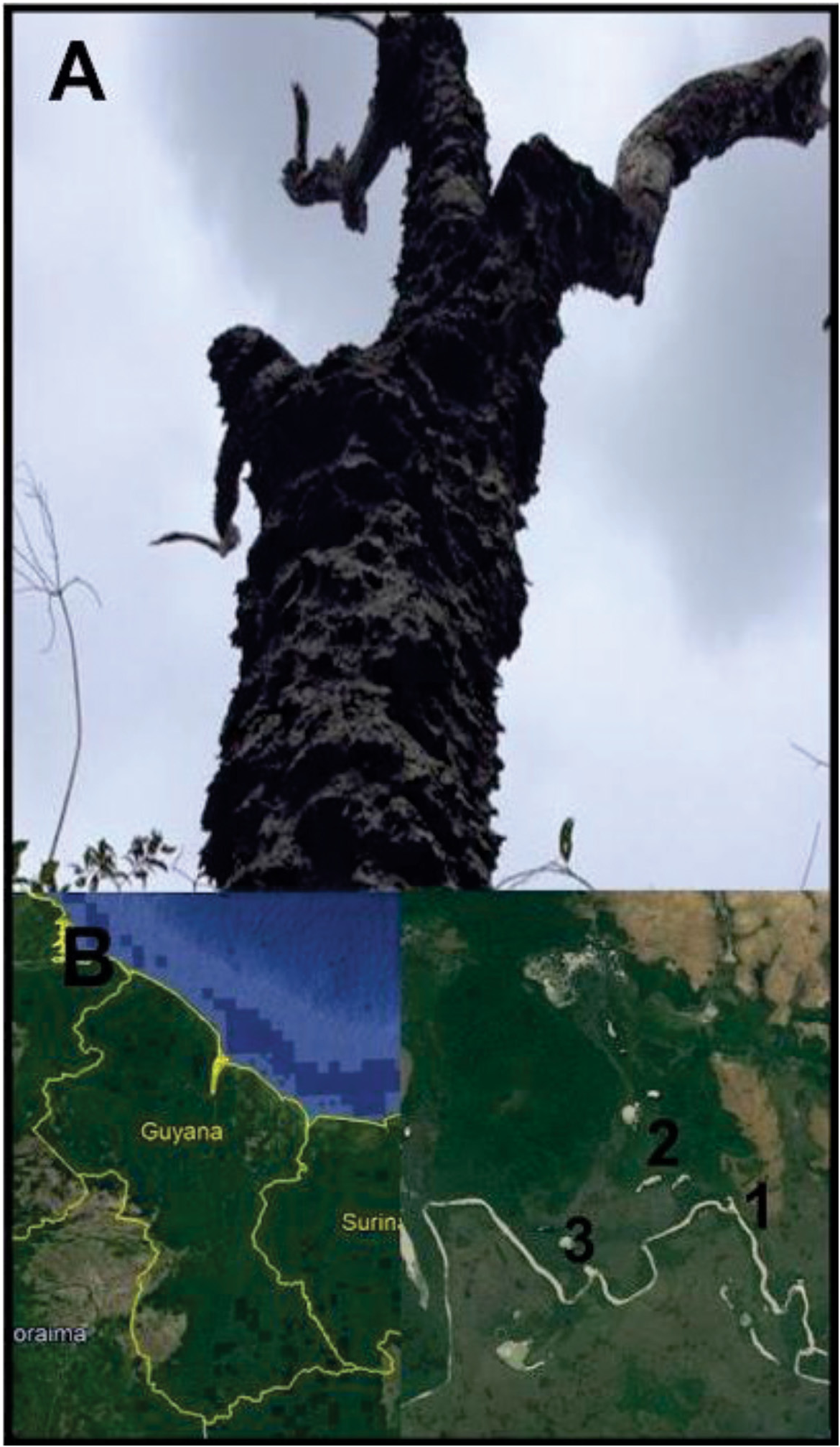
Locality: South America, Guyana: Rupununi River Region, Karanambu Ranch, Capuchin trail, elevation: 100km, 03°44.85’ N,059°19.13’ W, tropical rainforest surrounded by open savannah, on host termite in an arboreal soil mound, 11, January 2016, Collected by M. Wilson, J. Ware, P. Barden, S. George. L. Johnson, S.T. Mafla-Mills. GoogleMaps
On Amitermes sp. , preserved in ethanol. Deposited at AMNH and registered at NEMU Herbarium ( NEMU), labeled: South America, Guyana: Rupununi River Region, Karanambu Ranch , Capuchin trail , elevation: 100km, 03°44.85’ N, 059°19.13’ W, collected 11, January 2016, ( holotype: T1-P1S7S3 !, GoogleMaps isotypes: T2-P1S7S3 !, T2-P2S7S3 !).
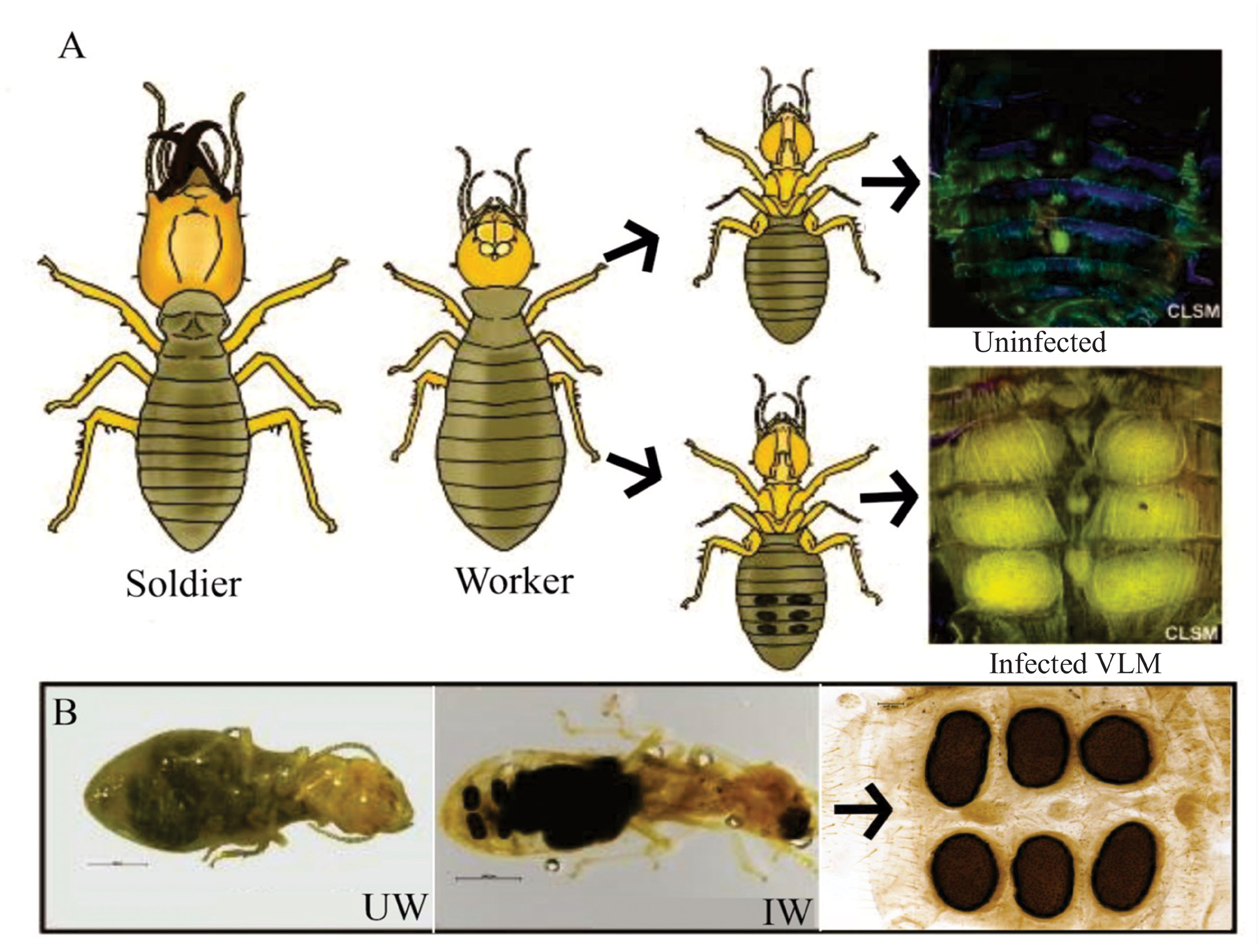
Diagnosis— Termitaria hexasporodochia sp. nov. exhibits a unique phenotype, exclusively forming six moderately sized black rimmed, elliptical sporodochia on the ventral abdominal segments (4–6) of its host. Termitaria hexasporodochia sp.nov. exhibits8–9conidia per collarette,is 116–120μm thick,elliptical in shape, has a conidiogenesis zone location 20μm–25μm from the sporodochium base, and thick-walled, lobate haustoria. The dimensions of the sporodochia range from 256 μm–609μm to 102–218 μm, the phialides are 110–115 μm in length, and the expiculum is the only pigmented region noted.
Description of the holotype —Entomogenous: Sporodochium thallus is 116 μm thick, 256 μm long, 102 μm wide, and ellipsoid in shape. At the basal layer, haustoria are initially formed from thick-walled haustorial mother cells at the base of the sporodochium and appear thick and lobular. Haustoria range from 19–23μm in length, and 1.7–2.4 μm in width. Superficial to this layer is the sporogenous hymenium layer composed of a mass of tight columnar phialides 110–115 μm thick. Each individual phialide ranges from 1.5–2.2 μm in diameter, terminating in two flat rounded tips. Endogenously-formed conidia originate from long rod-shaped conidiogenous cells at the conidiogenous locus, located 20–25 μm from the base of each phialide. Conidial spores are rectangular and catenate, 1–1.3 μm in width and 3–4 μm in length. 8–9 spores were found in each collarette and 11–13 in each phialide. Spores break off simultaneously as they reach the sporodochium surface. The surface of each sporodochial lesion appears perforated, bearing a hexagonal pattern referred to as “textura angularis”, with pores being larger in diameter along the rim preceding the sterile expiculum. The expiculum forms a smooth black crust with no apparent openings. The holotype selected for description was one of the six lesions found infesting the 4 th ventral abdominal segment of an Amitermes termite worker T1-S7S3!.
Etymology— Termitaria is the established generic name, and hexasporodochia is an adjective referring to the hexad arrangement of sporodochia exhibited on the type-host, Amitermes .
Ecology and host species— Amitermes sp. found to be infested with Termitaria were collected from large arboreal mounds constructed on sandpaper trees ( Curatella americana ) growing at the interface between open savannah and rainforest ( Fig. 2 View FIGURE 2 ). The mounds were found during the dry season (January) in regions surrounding the Rupununi River that flood during the annual wet season (May-August). It was found only on worker caste termite hosts, never on soldiers or reproductives.
Differential diagnoses— Termitaria hexasporodochia sp. nov. exhibits a unique arrangement phenotype, forming six moderately-sized, black-rimmed, elliptical sporodochia on the ventral abdominal segments (4–6) of its host. This fungus belongs to the genus Termitaria and is readily distinguished from two described members of the genus Mattirolella . It lacks a major generic character shared amongst Mattirolella , the sterile hyphae separating fertile hyphae in the hymenium is not present in the new species. The maximum lengths of T. hexasporodochia sp. nov. sporodochia are less than half the size of those described for Australian species, T. macrospora and T. rhombicarpa ( Table 1 View TABLE 1 ; Kimbrough & Lenz 1982). Additionally, T. rhombicarpa can be distinguished by the rhomboid shaped sporodochium it typically forms on the host as opposed to the elliptical/circular lesion formed on T. hexasporodochia sp. nov., shorter phialide lengths ( Table 1 View TABLE 1 , 100–105 μm vs. 110–115μm), longer conidiogenous locus ( Table 1 View TABLE 1 . 30–35 μm vs. 20–15 μm from phialide base) and haustoria penetrating twice as deep into the host cuticle ( TABLE 1 View TABLE 1 . 50–55 μm vs. 20–23 μm). T. hexasporodochia sp. nov. spores are easily distinguished from those produced by T. macrospora .
As its name suggests T. macrospora produces 4–5 massive spores per collarette (app 3.8–9.1 μm) whereas T. hexasporodochia sp. nov. produces 8–9 spores per collarette (app 1–1.3 × 3–4 μm). Another Australian species, T. longiphialidis , is best identified by its small circular sporodochia and having the longest phialides of any Termitaria species ( Table 1 View TABLE 1 . 160–180 μm vs. 110–115 μm; Kimbrough & Lenz 1982). T. hexasporodochia sp. nov. is readily distinguished from the commonly described T. snyderi , by phialide length. T. hexasporodochia sp. nov. possesses a much thicker sporodochium, with phialides double the length of those found in T. snyderi ( Table 1 View TABLE 1 , 110–115 μm vs. 50–60 μm). T. coronata can be distinguished from all Termitaria species, T. hexasporodochia sp. nov. included, in the appearance of its sporodochium, with its echinulate surface and the high position of its conidiogenous locus ( Table 1 View TABLE 1 , 50–60 μm vs. 20–25 μm).
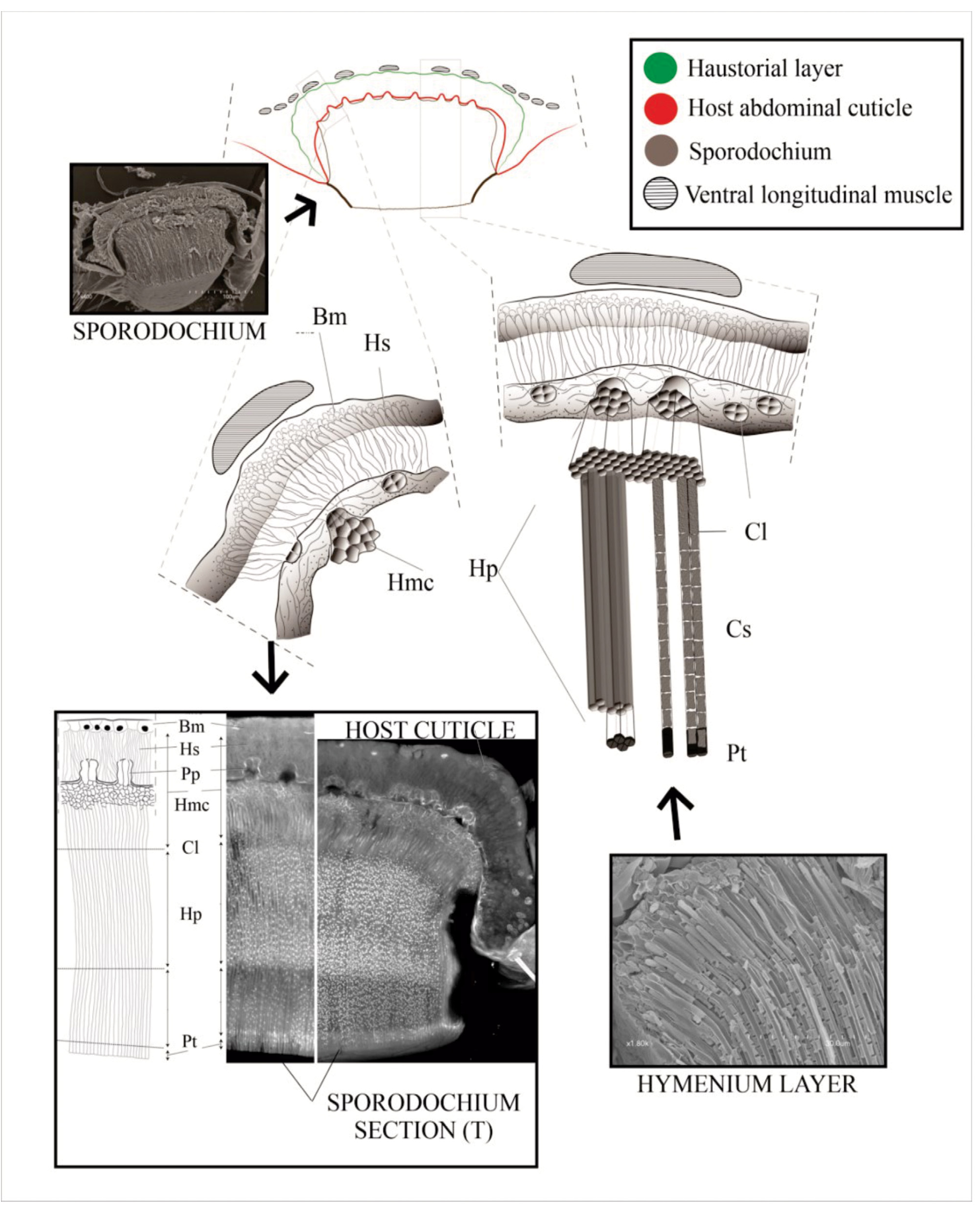
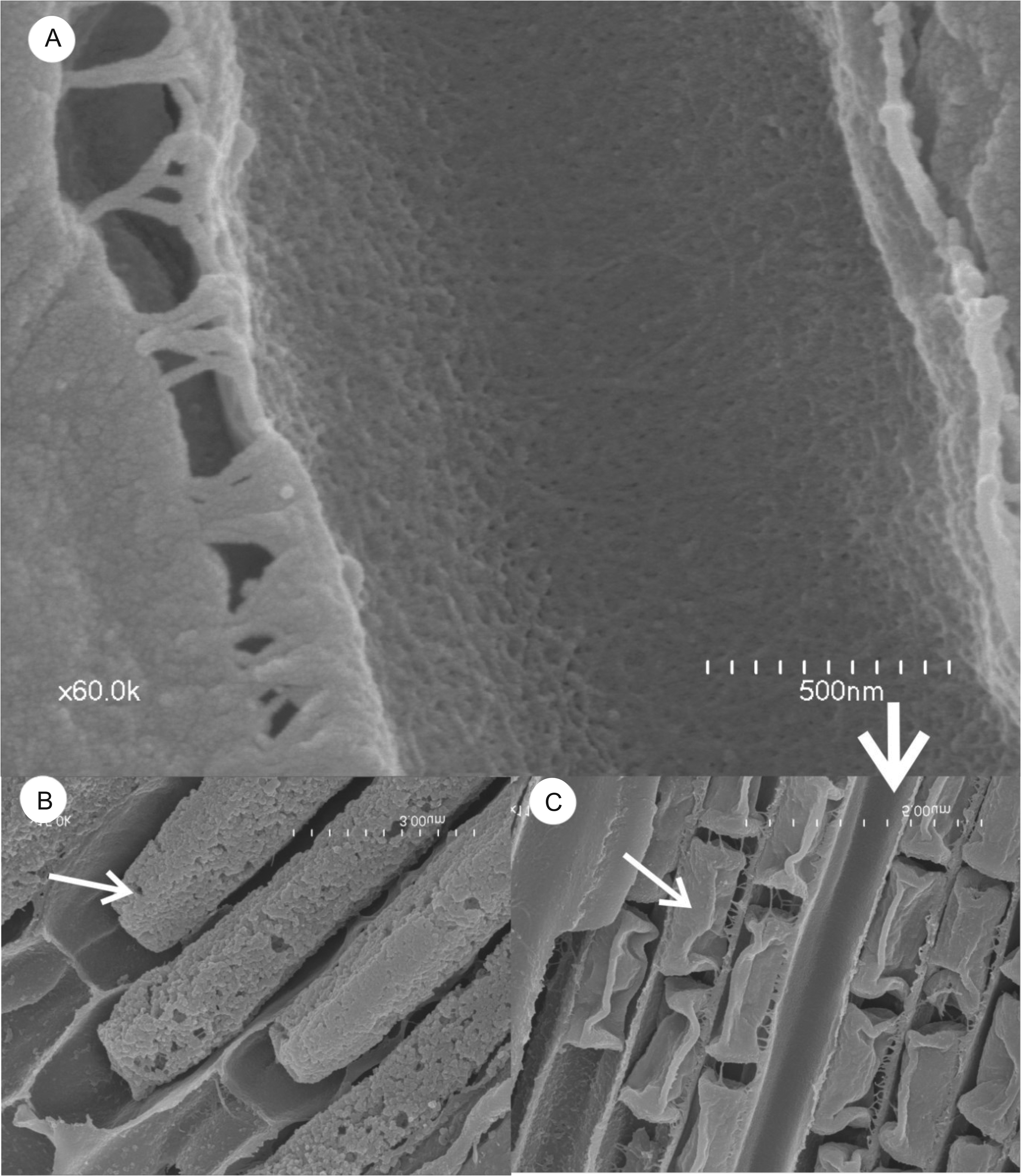
Ultrastructure of T. hexasporodochia sp. nov. —The complete sporodochium ( Fig.4 View FIGURE 4 ) is composed of many tightly aligned vertical columns. We group the sporodochia layers into three major regions ( Fig.5A View FIGURE 5 ); the basal region ( Fig. 5 View FIGURE 5 ) most closely appressed to the insect cuticle, the sporogenous hymenial region ( Fig. 6 View FIGURE 6 ), and the upper region ( Fig.7 View FIGURE 7 ) filled with conidia and phialides terminating into flaps.
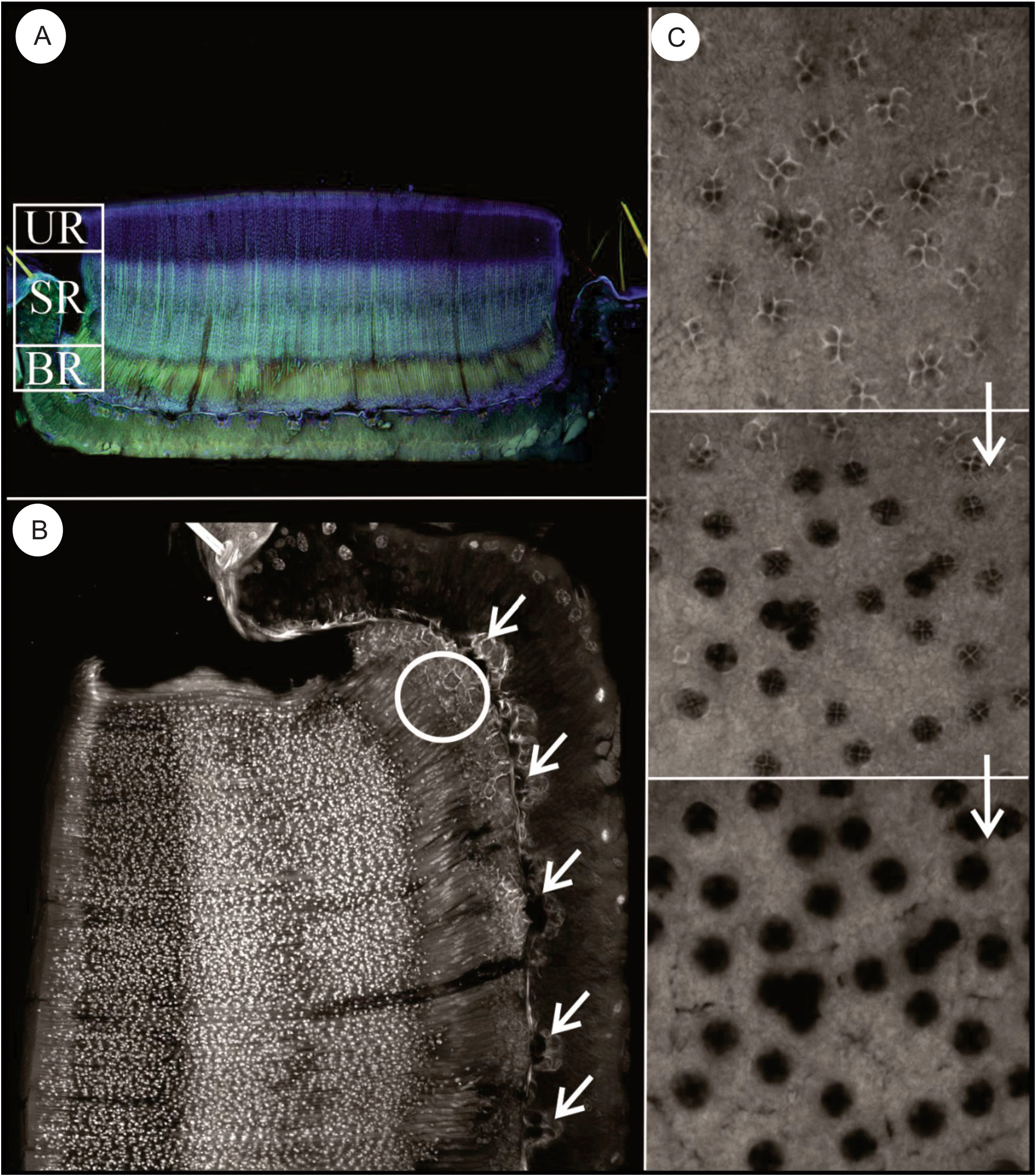
The basal region —This region of the sporodochium is approximately 4–5 rows and 8.5–12.5 μm thick. It is comprised of haustorial mother cells that give rise to a subcuticular layer of haustoria that penetrates the host ( Fig. 5 View FIGURE 5 ). The thin-walled cells composing the upper layer of the basal region have been referred to in previous studies as the “subhymenial layer” and gives rise apically to the hymenial phialides ( Kimbrough and Thorne 1982). Haustoria aggregate towards and penetrate the host via tetra ocular channels in its cuticle. We observe 12+ major penetration points between the fungal body and its host ( Fig.5 B View FIGURE 5 ), extending below the cuticle to thick-walled lobular haustoria ranging from 19–23 μm in length and 1.7–2.5 μm in width. We do not refer to this “haustorial region” as an internal region within the sporodochium because it lays below the cuticular layer of its host ( Fig.6E–F View FIGURE 6 ). Additionally, its penetration into the host cuticle does not change the classification of this fungus as an ectoparasite, in that it does not invade the host cytoplasm. Each sporodochia appears to be formed from major infestation sites with cells growing upward from the basal layer, with approximately 160–240 openings per sporodochium (size dependent) that appear dark in photomicrographs ( Fig.5C View FIGURE 5 ).
The sporogenous hymenium region —This region consists of a thick hymenium of tightly appressed phialides ( Fig.6A View FIGURE 6 ), comprised of long rod-shaped conidiogenous cells 14–22 μm in length, which differentiate into asexual conidia at a fixed conidiogenous locus ( Fig 6B–C View FIGURE 6 ). The phialides are approximately 1.5–1.2 μm in diameter and the conidiogenous locus ( Fig.6D View FIGURE 6 ), the initial point of asexual spore differentiation, occurs 20–25μm, from the base of each hymenial phialide. Each collarette contains 8–9 rectangular, catenate conidia ( Fig.7B–C View FIGURE 7 ), and approximately 11–13 spores can be found in the phialide during this time. Under high-definition microscopy, the internal surface of the hymenial phialides are coated with a dense mat of minute filaments that appear to be restricted to the upper 3/4th of the secondary canals ( Fig.7A View FIGURE 7 ).
The upper region — At the superficial level of the sporodochia, a dark, peripheral expiculum surrounds a region resembling a hexagonal honeycomb ( Fig.7A,C View FIGURE 7 ). This pattern is formed by fields of terminating phialides ( Fig.3B View FIGURE 3 ) of elongate tubes referred to as textura angularis, the apices of which are bivalved ( Fig 7B–D View FIGURE 7 ). The apical valves are formed by two isosceles trapezoidal flaps that fit closely together to form a large circular pad of thousands of hexagons ( Fig.7D View FIGURE 7 ). Visible from confocal stack images just below the pad surface, each sporodochia appears densely populated with hexagonal pores, with the conidial spores visible within each tubular hymenial channel leading to the apical pore valves ( Fig.7B View FIGURE 7 ). On average, a sporodochia contains approximately 12,000 –14,000 phialides.
Overall, the most striking feature of Termitaria hexasporodochia sp. nov. is apparent in the arrangement and ultrastructure of the six moderately-sized, black-rimmed, elliptical sporodochia it forms on its termite host. Of all members of the family Kathistaceae , the formation of sporodochia on termites is diagnostic for members of the genera Termitaria and Mattirolella , hence why we focus on those two genera in assessing the new species. Termitariopsis is the only remaining member of Kathistaceae which forms sporodochia, although it does not use termites as a host and exhibits sporodochial features absent in the other two genera ( Table 2a View TABLE 2 ). Kathistes genera do not form sporodochia and are described instead off of their sexual form, whereas Termitaria hexasporodochia sp. nov. is described by its asexual sporodochia form. The genus Ectomyces calotermi , described on termites to form sporodochial lesions, has been synonymized with Termitaria snyderi ( Tate 1928) , a species described in this article. No further examination is needed on this genus as its synonym T. snyderi is examined here. We place the new species in Termitaria because it lacks the defining features of Mattirolella : sterile hyphae interspersed with fertile hyphae and an epihymenium ( Table 2a View TABLE 2 ). The new species can be differentiated from other Termitaria species by sporodochial length ( T. macrospora and T. rhombicarpa ), sporodochial shape ( T. rhombicarpa ), phialide lengths ( T. longiphialidis , T. snyderi ), location of conidiogenous locus, spore size, and haustorial depth ( Table 2a View TABLE 2 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |