Amapacylapus Carvalho & Fontes, 1968
|
publication ID |
https://doi.org/10.1515/aemnp-2017-0084 |
|
publication LSID |
lsid:zoobank.org:pub:03305E03-AF44-4C6D-9E2B-9A3EE979C5AF |
|
persistent identifier |
https://treatment.plazi.org/id/03E287C9-F45F-FFEA-FECF-FC46CF5EFC18 |
|
treatment provided by |
Marcus |
|
scientific name |
Amapacylapus Carvalho & Fontes, 1968 |
| status |
|
Amapacylapus Carvalho & Fontes, 1968 View in CoL View at ENA
( Figs 1–26)
Amapacylapus Carvalho & Fontes, 1968: 279 View in CoL (new genus). Type species: Amapacylapus amapariensis Carvalho & Fontes 1968 View in CoL (original designation).
Amapacylapus: CARVALHO & FROESCHNER (1987) View in CoL : 125 (list); SCHUH (1995): 19 (catalog); GORCZYCA (2000): 48 (list); GORCZYCA (2006b): 13 (catalog); SCHUH (2013) (online catalog); NAMYATOVA et al. (2016): 5, 24, 32, 33 (as outgroup in phylogenetic analysis of the subfamily Bryocorinae ).
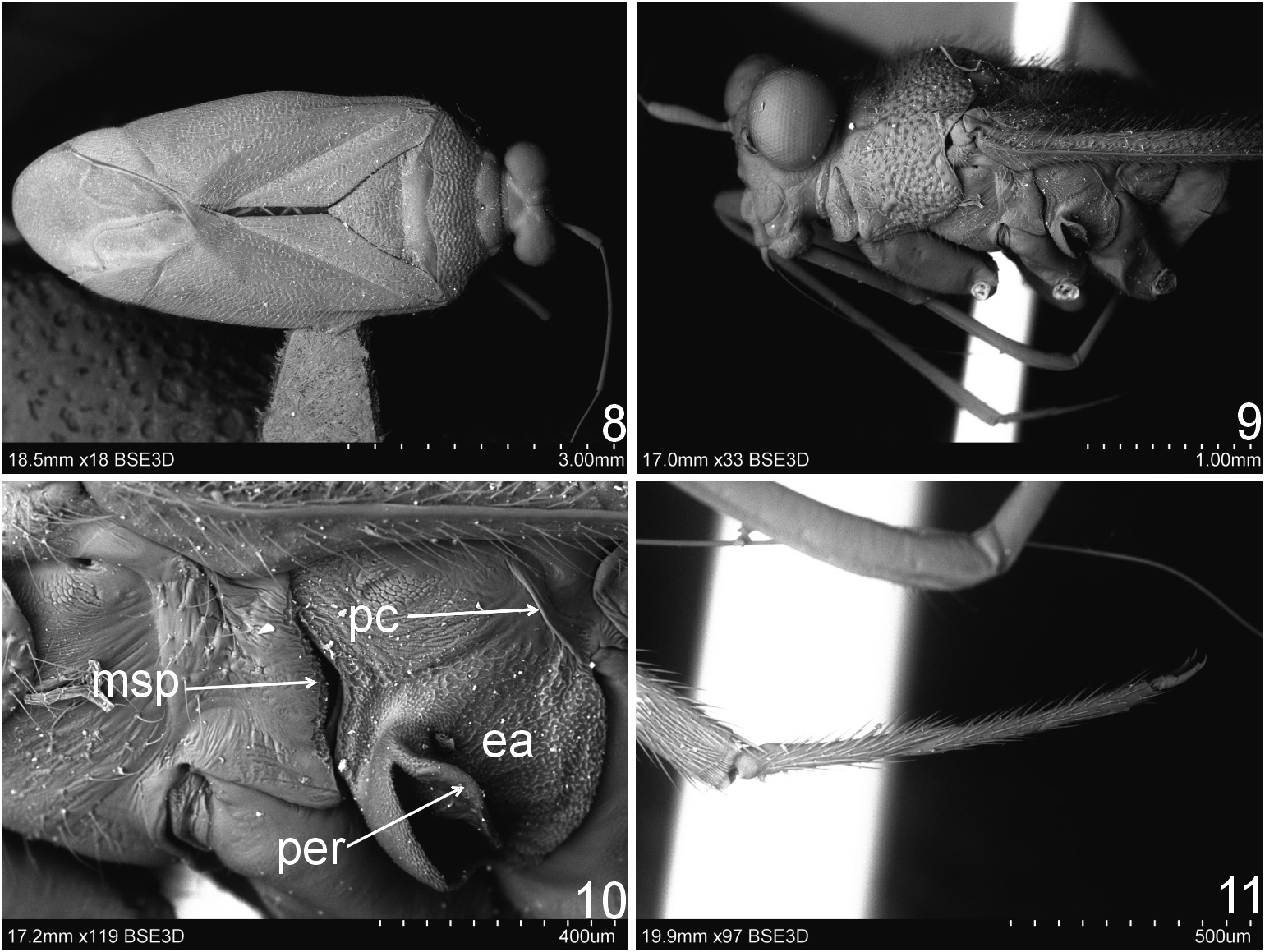
Diagnosis. Recognized by the following set of characters: metathoracic scent gland with ostiolar canal broad, strongly raised above surface of evaporative area and peritreme narrow ( Fig. 10 View Figs 8–11 , arrow); corium with regular yellow pattern (see generic description) ( Figs 1–5); tarsomere I about two times shorter than tarsomeres II and III ( Fig. 11 View Figs 8–11 ); endosoma with one sclerite ( Figs 12, 18 View Figs 12–22 ).
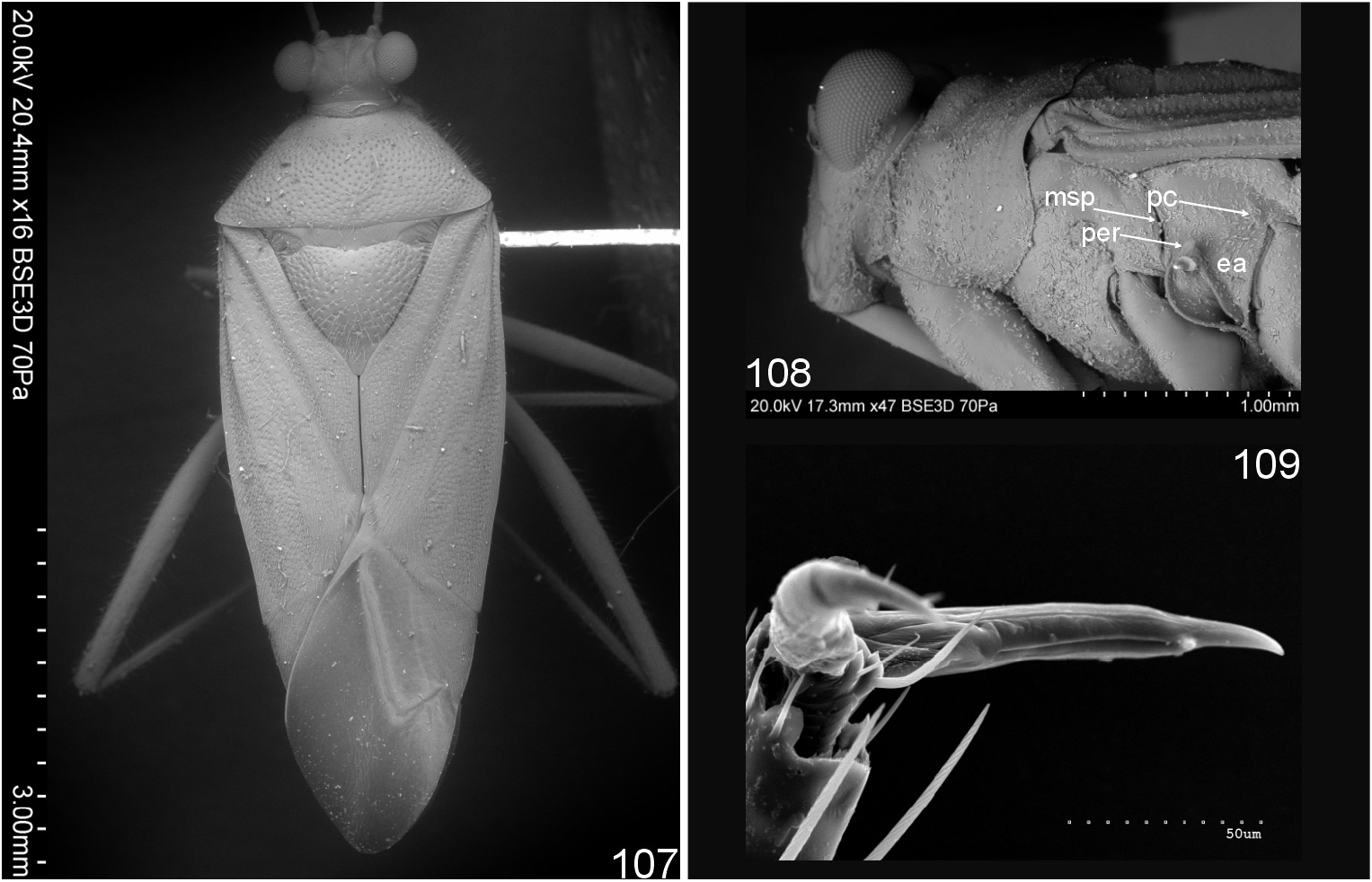
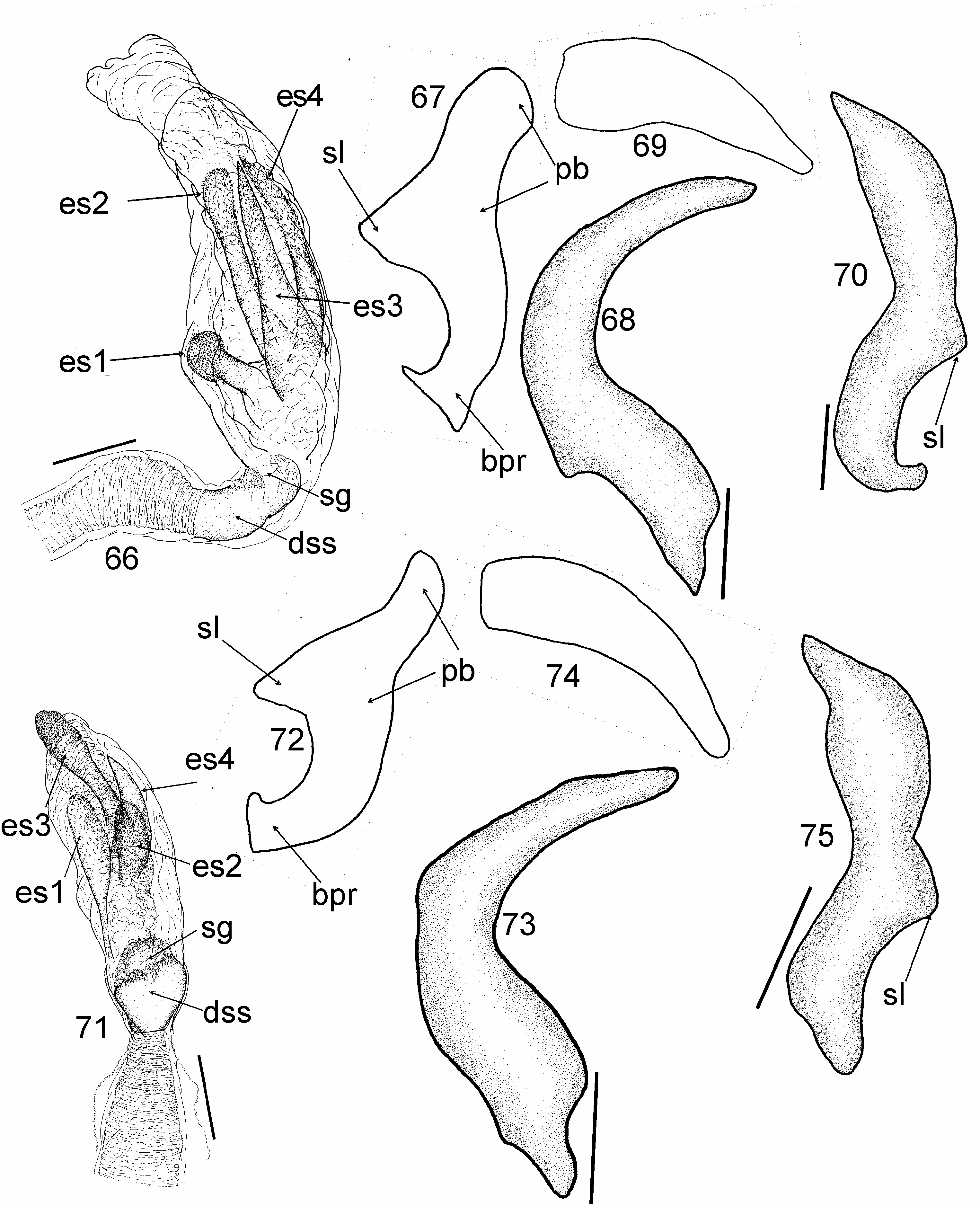
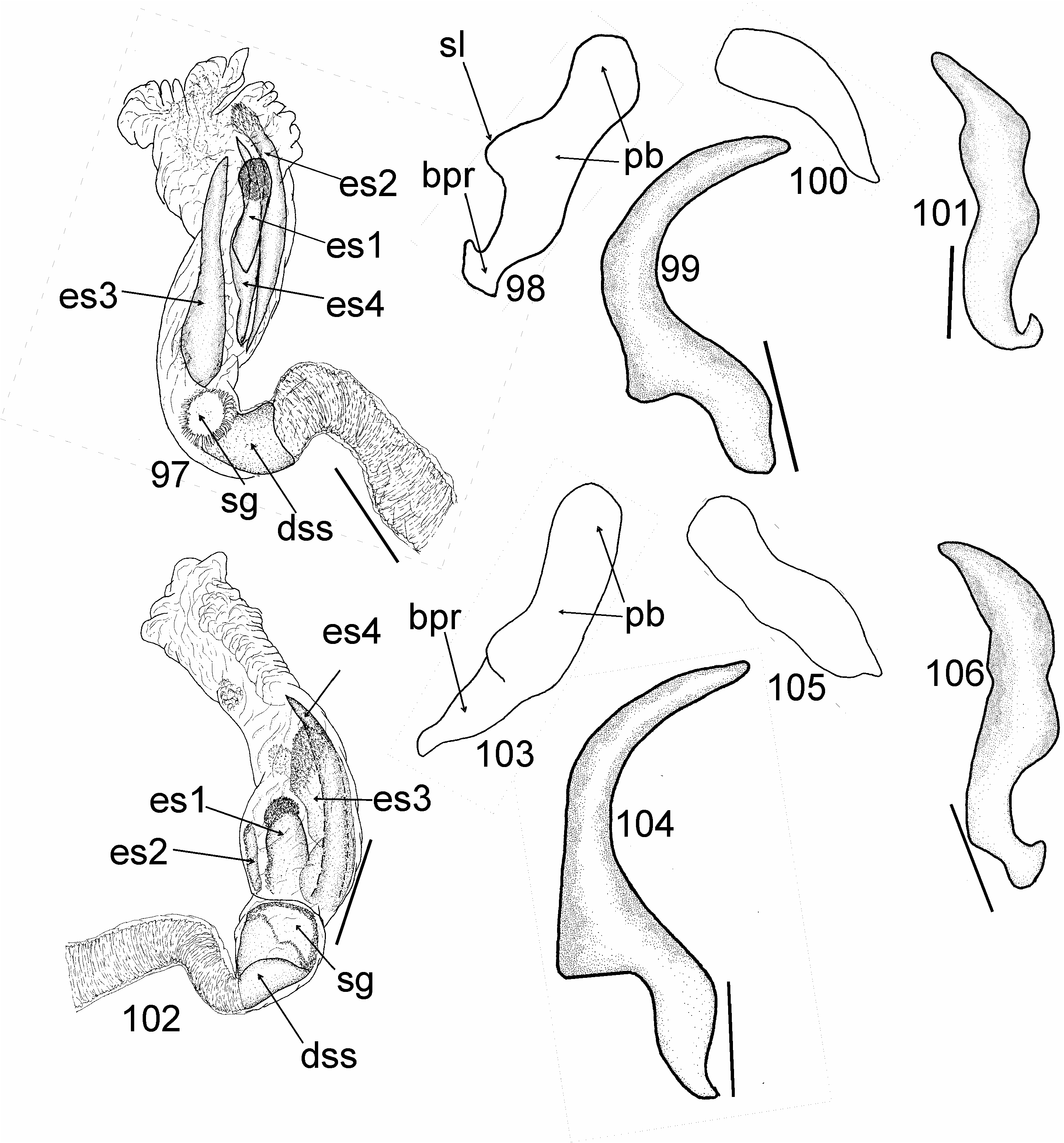
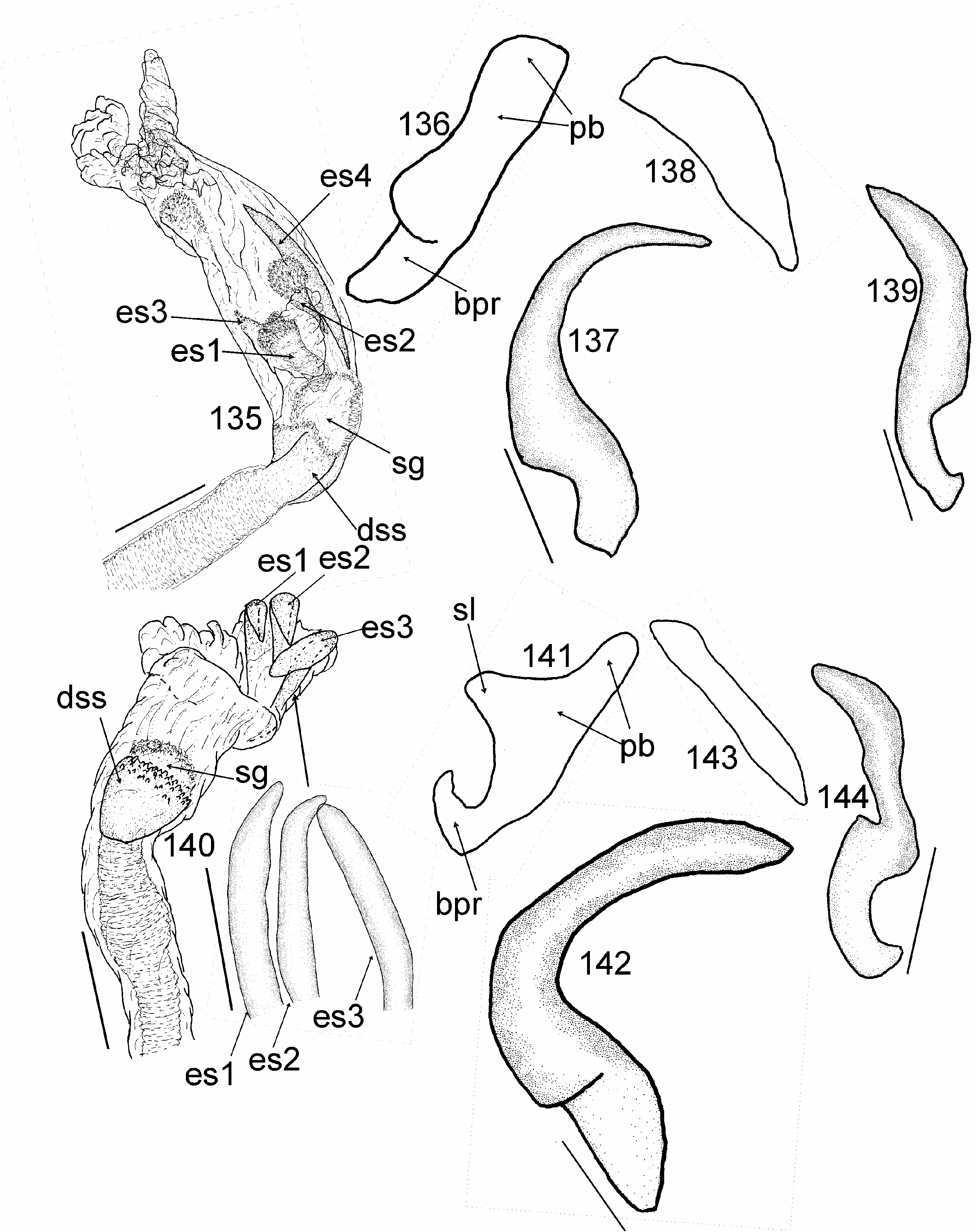
Most similar to Cylapus (as diagnosed herein) in sharing the ostiolar canal strongly developed, raised above evaporative areas (cf. Fig 10 View Figs 8–11 with Figs 93, 96, 108 View Figs 107–109 , 129, 146 View Figs 146–151 ). It can, however, be distinguished by the reduced metathoracic scent gland peritreme (well developed in Cylapus ) (cf. Fig 10 View Figs 8–11 with Figs 93, 96, 108 View Figs 107–109 , 129, 146 View Figs 146–151 , arrow), the tarsomere I about two times shorter than tarsomeres II and III combined (tarsomere I as long as or longer from II and III combined in Cylapus ) (cf. Fig 10 View Figs 8–11 with Figs 94, 132), and by having of the endosoma with a single sclerite (usually at least two sclerites in Cylapus ) (cf. Figs 12, 18 View Figs 12–22 with Figs 66, 71 View Figs 66–75 , 80, 85 View Figs 80–89 , 97, 102 View Figs 97–106 , 110 View Figs 110–119 , 135, 140 View Figs 135–144 ).
Redescription. Coloration. ( Figs 1–7, 23–26 View Figs 23–26 ). Dorsum dark brown with distinct yellow or dirty yellow areas. Thorax. Mesoscutum and scutellum. Mesoscutum fuscous, often with two yellow stripes each situated laterally and contiguous with basolateral patches on scutellum; scutellum fuscous with two large, yellow patches each situated basolaterally, medial portion with longitudinal, yellow stripe, apex with yellow patch. Hemelytron. Dark brown to fuscous with yellow pattern, when fully developed composed of seven patches: one on basal portion of R+M vein (p1), two situated near base on exo- and endocorium (p2, 3), one situtated apically on endocorium (p4), one on outer, apical angle of exocorium (p5), one on inner, apical angle of endocorium bordering membrane (p6), and one on inner, apical angle of endocorium situated near apex of clavus (p7); clavus with yellow patch basally and apically; cuneus with more or less developed yellow patch on basal margin; membrane fuscous two yellow patches: one, larger situated medially, bordering or nearly bordering membrane major cell and other contiguous with inner margin of cuneus. Legs. Brown to black; tibiae with at least one, contrastingly yellow annulation.
Structure, texture and vestiture ( Figs 1–11, 23–26 View Figs 23–26 ). Macropterous. Body elongate oval; dorsum punctate, mixed with relatively long, dense, erect and semirecumbent setae.
Head. Eyes strongly pedunculate; vertex ecarinate posteriorly, medial sulcus of vertex deep; antennal segment I shorter than width of head, narrowed basally, rest of the segment nearly cylindrical, weakly broadened medially; segment II about two times thinner than segment I, cylindrical. Thorax. Pronotum. Posterior margin convex medially. Mesoscutum and scutellum. Mesoscutum well exposed; scutellum moderately convex. Thoracic pleura impunctate, shiny, covered with sparse, long, erect setae; mesepimeral spiracle indistinct, surrounded by mushroom bodies; metathoracic peritreme strongly raised above evaporative areas, strongly reduced, ostiolar canal distinctly developed and raised above surface of evaporative areas, tubelike. Hemelytron. Outer margin moderately arcuate. Male genitalia ( Figs 12–22 View Figs 12–22 ). Aedeagus ( Figs 12, 18 View Figs 12–22 ). Endosoma strongly membranous with single sclerite; secondary gonopore broad, irregularly shaped, strongly serrate, directed upwards; sclerotized portion of ductus seminis inside endosoma short. Right paramere ( Figs 16, 22 View Figs 12–22 ) sickle shaped; apical process relatively long.
Discussion. CARVALHO & FONTES (1968) recognized Amapacylapus based on the following characters: the presence of the furrow on the maxillary plate, thin antenna with sparse setae, short antennal segment I broadened toward apex, and strongly punctate body. These characters do not uniquely distinguish Amapacylapus as they are quite common among members of Cylapini . Examination of A. amapariensis , the type species, and two additional species, A. englemani Carvalho, 1991 and A. unicolor sp. nov. reveal there are two stable features that strongly indicate their close similarity and are here treated as diagnostic for Amapacylapus : hemelytron dark brown to black with regular yellow pattern composed of yellow patches situated on basal, medial, and apical parts of exo- and endocorium, apical part of clavus, and two patches on membrane: one bordering distal angle of major cell and other bordering inner margin of cuneus ( Figs 1–5, 23–26 View Figs 23–26 , arrows) and peritreme narrow, strongly raised from the surface of metepisternum ( Figs 10 View Figs 8–11 , arrow). Identical coloration of the hemelytron is found in Peltidocylapus labeculosus (see holotype photo at ANONYMOUS 2017 and reproduced in Fig. 4 of this paper). Careful examination of the metathoracic scent gland peritreme and other body elements of this type specimen were not possible. However, based on its strong resemblance to the type species of A. amapariensis it seems clear that P. labeculosus belongs to Amapacylapus . Thus, I propose to transfer this species from Peltidocylapus to Amapacylapus .
The hemelytral coloration is significantly different in two species, A. nigricapitis Carvalho, 1986 and A. rondoniensis Carvalho, 1986 , included in Amapacylapus by CARVALHO (1986). The type specimens of both species were unavailable for study but according to CARVALHO (1986) the hemelytron is either entirely black ( A. rondoniensis ) or dark castaneous with costal fracture yellow ( A. nigricapitis ), and in both species the membrane is entirely fuscous which would exclude them from Amapacylapus as diagnosed in the present paper. CARVALHO (1986) did not provide a detailed description of the metathoracic scent efferent system, mentioning only the distinctly raised ostiolar peritreme in A. rondoniensis ; placement of A. rondoniensis and A. nigricapitis cannot be judged based on this body part.
Among the New World Cylapini only some members of three genera, i.e. Cylapus (as diagnosed in this paper), Peltidocylapus , and Valdasus , possess uniformly dark, dark brown to black coloration (e.g., Figs 36–38 View Figs 36–40 , 44, 45). According to CARVALHO (1986: Fig. 4) the endosoma in A. rondoniensis and A. nigricapitis are devoid of sclerites. Taking this into account the placement of both species in Cylapus can possibly be excluded as most species have at least three distinct sclerites ( Figs 66, 71 View Figs 66–75 , 80, 85 View Figs 80–89 , 97, 102 View Figs 97–106 , 110 View Figs 110–119 , 135, 140 View Figs 135–144 ). Endosoma without sclerites can be found in most members of Peltidocylapus (Wolski, in prep.), which may indicate a placement of A. rondoniensis and A. nigricapitis in this genus. Detailed studies of specimens belonging to both species, including examination of the type specimens are required to confirm their generic affiliation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Amapacylapus Carvalho & Fontes, 1968
| Wolski, Andrzej 2017 |
Amapacylapus :
| GORCZYCA J. 2006: 13 |
| GORCZYCA J. 2000: 48 |
| SCHUH R. T. 1995: 19 |
| CARVALHO J. C. M. & FROESCHNER R. C. 1987: 125 |
Amapacylapus
| CARVALHO J. C. M. & FONTES A. V. 1968: 279 |