Hyla heinzsteinitzi, Grach & Plesser & Werner, 2007
|
publication ID |
https://doi.org/ 10.1080/00222930701261794 |
|
persistent identifier |
https://treatment.plazi.org/id/03E3FD30-FFCC-9F47-C507-FC7B4BFDFA28 |
|
treatment provided by |
Felipe |
|
scientific name |
Hyla heinzsteinitzi |
| status |
sp. nov. |
Hyla heinzsteinitzi View in CoL sp. n.
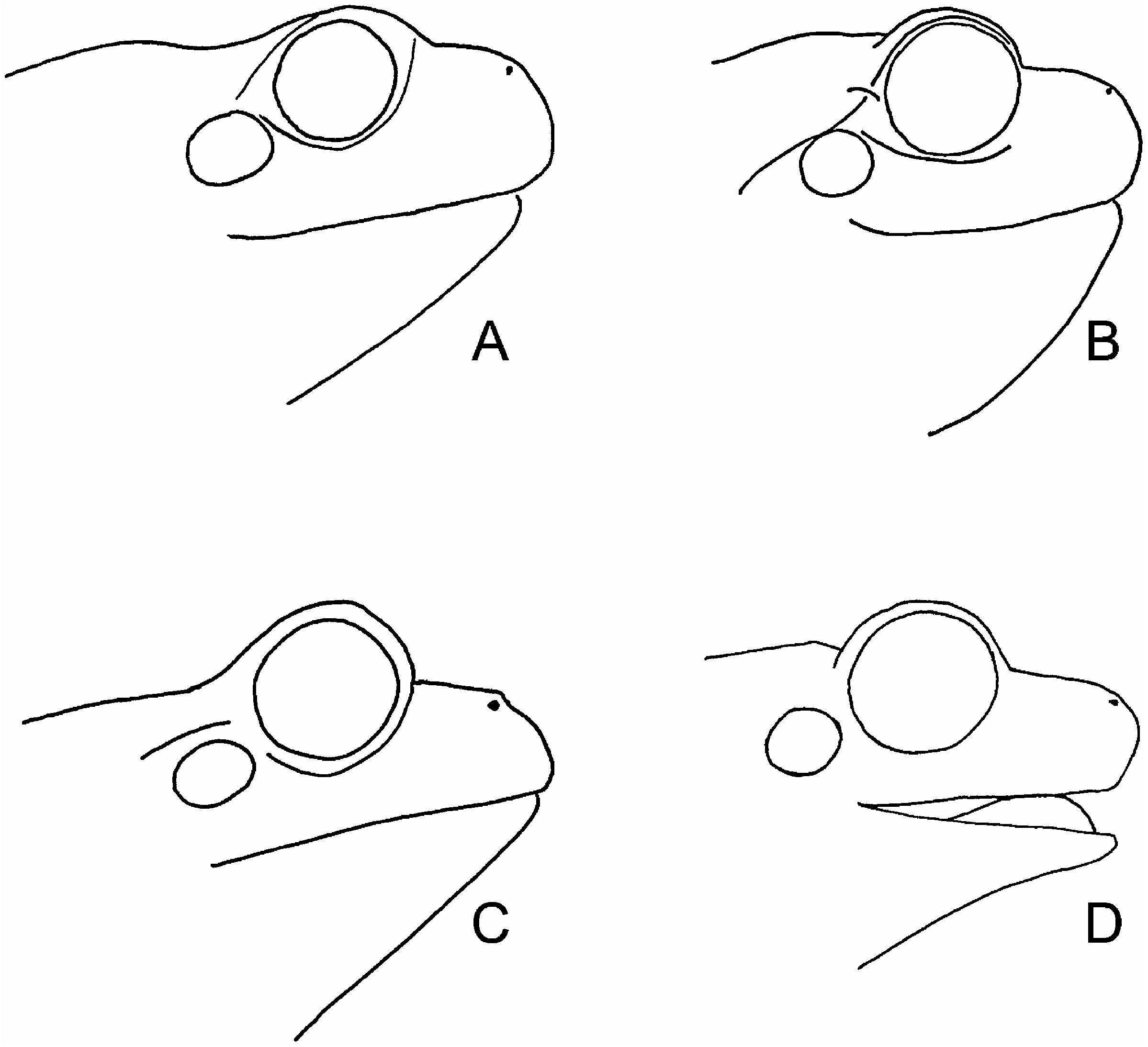
( Figures 1 View Figure 1 and 3 View Figure 3 )
Hyla arborea (Linnaeus, 1758) View in CoL (part): Tristram 1885, pp.160–161; Lulav 1978, p. 431; Fishelson 1979, p. 327.
Hyla arborea var. savignyi Audouin View in CoL , ‘‘1809’’ [1827], p. 183; 1829, pp. 137–138 (part): Boulenger 1882, p. 380; 1898, pp. 250–251; Anderson 1898, pp. 357–358.
Hyla arborea savignyi Audouin, 1827 View in CoL (part): Flower 1933, pp. 843–844; Bodenheimer 1937, p. 73; Aharoni p. 1940, p. 56; Mendelssohn and Steinitz 1944, p. 294 –295.
Hyla arborea savignyi Audouin, 1827 View in CoL : Shy 1980, 1985.
Hyla savignyi View in CoL (part): Bodenheimer 1935, p. 199; Schneider and Nevo 1972; Werner 1988, p. 359, 1995, pp. 12–15; Bouskila and Amitai 2001, pp. 32–34; Disi et al. 2001, pp. 97–98.
Type series
Holotype. HUJ-R 20193 , female, Mamilla reservoir, IG 1710 1317, Jerusalem, Israel, spring 1996, C. Grach (collected as tadpole, raised in captivity, died accidentally when two years old).
Paratypes (n 512). HUJ-R 15186 , 15188–15190 , 15192 , 15194 males, Wadi near Moza, Judaean Hills at IG 1658 1353, April 1977, E. Shy ; HUJ-R 15193 male, Ein Fara , Wadi El Qilt, Judaean Desert , IG 1789 1378, approx. 300 m a. s. l., October 1976, E. Shy ; HUJ-R 15187 male, 15185, 15191 females, either of the previous two locations ; HUJ-R 20198 female, 20199 male, captive bred spring 2000, from parents collected as tadpoles from the
Mamilla reservoir ( IG 1710 1317, Jerusalem, Spring 1996, C. Grach) and raised in captivity.
Differential diagnosis
A tree frog of the Hyla arborea group differing from all others in the structure of the call, in which the energy amplitude has a short rise time and a much longer decay time. Morphologically its snout has a truncate, rather than round, profile; the dark lateral stripe, visible in daytime along the flank, lacks the inguinal branch of H. arborea and is strongly fragmented. The white stripe along the lips is greatly reduced, anteriorly absent. Apparently endemic to the Judaean Hills, H. heinzsteinitzi differs from sympatric H. savignyi (values in parentheses) especially in the shorter eye–snout distance,,8 PERCRA (.9 PERCRA) with greater nostril-lip distance, approx. 7 PERCRA (approx. 6 PERCRA); wider interorbital space, at minimum.25 PERCRA (,23 PERCRA); and somewhat coarser feet, with longer callus internus,.4 PERCRA (,4 PERCRA) and larger digital discs, that of the third finger approx. 4 PERCRA (approx. 3 PERCRA).
Description of the holotype
Adult female. The radiograph showed the following skeletal characters which are typical of the Hylidae ( Nieden 1923) and were identical in three cleared and stained control specimens, Israeli H. savignyi : vertebrae procoelous, no free ribs, sacral transverse process broad, urostyle connected with two joints, all fingers and toes with the terminal phalanx claw-like.
External morphology. Head distinctly wider than long, snout acutely rounded in dorsal view, moderately truncate in profile; top of head flat (between nostrils and between eyes); nostril vertically elliptical, facing laterad; eye much larger than tympanum, protruding, pupil horizontal, supratympanic fold distinct, starting at the eye, extending caudad to behind axilla; upper jaw toothed, palate toothed; body and limbs without skin folds; skin of dorsum smooth, of venter glandular (granular), of throat intermediate; heels of adpressed hindlimbs meet or overlap by up to half the diameter of the eye; all fingers and toes with terminal digital pads; only the feet webbed, with thin webs between toes 2–5, webbing formula II 3-2 III 3-1 IV 1–2 V.
Measurements. In mm ( PERCRA) and where relevant left/right: ra 44 mm; HLj 10/9.7 (22.7/22.05); HLt 10.8/10.1 (24.5/22.05); HW 13.7 (31.1); IND 2.9 (6.6); IOM 10.5 (23.9); IOA 7.9 (17.9), IOP 11.5 (23.1); ED 4.6/4.5 (10.4/10.2); TD 2.7/2.5 (6.1/5.7); NL 3.4/3.4 (7.7/7.7); ES 3.6/3.4 (8.2/7.7); EN 3.8/3.5 (8.6/8.0); ET 1.3/1.4 (3.0/3.2); TL 1.7/ 1.5 (3.9/3.4); FA 9.7/10.5 (22.05/23.9); FM 20.1/20.8 (45.7/47.3); TB 19.5/19.5 (44.3/ 44.3); TR 10.8/10.4 (24.5/23.6); T 4 16.8/16.6 (38.2/37.7); T 1 4.4/4.4 (10/10); C 1.9/1.7 (4.3/3.9); W 9.5 /9.1 (21.7/20.7); DD3 1.6/1.8 (3.6/4.1); DD4 1.7/1.4 (3.9/3.2).
Proportions. HW:HLj51.37/1.41; ES:EN50.95/0.97; TD:ED50.59/0.55; T 1: T 450.26/ 026; W: T 450.57/0.55.
Colouration in preservative. Generally grey. Upper lips only indistinctly and posteriorly with light stripe. Dark, somewhat brownish, lateral stripe, its upper and lower margins emphasized, extending from behind eye (above tympanum) to just behind axilla, more posteriorly represented by indistinct, interrupted, fragments of irregular shape; flanks and posterior half of dorsum with small roundish dark spots in lighter halos; small dark dots pepper the tibias. Ventral side ‘‘off-white’’.
Variation and comparisons
The qualitative morphological description holds true for the type series. In the control H. savignyi specimens the snout is less truncate, more rounded ( Figure 3 View Figure 3 ).
Principal Coordinates Analysis ( Figure 4 View Figure 4 ), based on 14 mensural characters marked in Table I, displayed two fairly distinct clusters when the individuals were identified by snout shape.
The mensural characters and their variation are compared in Table I with those of the control sample of sympatric H. savignyi . Both samples showed little asymmetry: in H. heinzsteinitzi the tympanum–lip distance was greater on the R (P 50.05), and the toe webbing greater on the L (P 50.03); in the control H. savignyi sample the ratio eye–snout: eye–nostril was greater on the R (P 50.02). These characters are treated in Table I separately for the R and L sides. Sexual dimorphism was significant only in the control H. savignyi sample: the distances nostril–lip and eye–snout were greater in the males (P 50.02, P 50.01 respectively). For practical purposes we show in Table I the variation of all characters within and between the two taxa, after pooling the sexes. In Israel there seems to be no sexual size difference in H. savignyi : the largest of 44 males measured 49.0 mm and the largest of 31 females 48.6 mm (Berger, Seligmann and Werner, unpublished). However, this seems to vary geographically: in Arabia, among 129 specimens, the largest male measured 42.0 mm and the largest female 47.5 (Balletto et al. 1985).
The colouration in most preserved specimens is rather faded. Yet in the H. savignyi sample the upper lips are consistently and conspicuously marked by a light or white stripe all around; in H. heinzsteinitzi this stripe is indistinct and limited to the posterior part of the lip.
Live frogs change colour through the diel cycle. In daytime the dark lateral stripe is distinct; at night it pales and may disappear. Additionally daytime colouration can change between green and various shades of brown and grey, with or without spots. The night colouration seems to vary with temperature, towards green when cold and brown when warmer. The ontogenetic development of these capacities for colour change seems to be completed only four months after metamorphosis (in captivity; in nature presumably at least six months). The following comments are based on the green phase of daytime colouration of frogs at least eight months old (see Material for description of samples).
In the H. heinzsteinitzi sample originating from the Mamilla reservoir the general body colour resembled turquoise A-5 or A-6 on p. 25 ( Kornerup and Wanscher 1978) and this accorded with the notes taken four years earlier of the parent generation. In contrast, the control animals and photographs of Hyla savignyi approximated B-7, B-8, C-7 or C-8 on p. 29, apple-green. The hidden parts of the legs, especially thighs, were orange in H. heinzsteinitzi and brown in H. savignyi .
The dark lateral band score of the H. heinzsteinitzi sample (see Material) ranged over 3–4 (completely represented by dots), with the median at 4. Thus it was generally fragmented. Often it resembled a broad belt of irregular spots, sometimes cloud-like, sometimes rosettelike. For the H. savignyi control sample the score ranged over 0–2, with the median at 1.75, nearly complete ( Figure 1 View Figure 1 ). In Arabia, according to the text of Balletto et al. (1985), the dark lateral band is complete to the groin but according to their photographs its ventral border tends to be ‘‘washed out’’ behind the axilla, and at least in one individual the band is a little fragmented in the groin.
In Israel our individuals of both samples scored symmetrically for the dark lateral band, except one in which the sides differed by one half-unit. Interestingly, in the sample from Cyprus, 11/ 25 specimens were asymmetrical in this respect. In a larger sample of 75 H. savignyi from Israel the lateral band was variable, in nine it had a partial inguinal branch bilaterally (in one or two fairly complete) but in eight this branch was unilateral. The occurrence of the inguinal branch was not correlated with morphometry and could not be defined geographically (Berger, Seligmann and Werner, unpublished) .
There is also a difference in the spotted phase. While in local Hyla savignyi , as in H. arborea , the background of grey, brownish, or yellowish may be spotted with darker or lighter blotches ( Boulenger 1897 –98; Nielsen 1980; Engelmann et al. 1993; Özeti and Yılmaz 1994; YLW pers. obs.), in H. heinzsteinitzi there exists an additional colouration, not known to us from local H. savignyi : brown (rust-brown, golden-brown) background, with green blotches.
Bioacoustics
The advertisement call of Hyla heinzsteinitzi resembles the published calls of H. savignyi and H. arborea in that it comprises a sequence of similar, evenly spaced, segments. Shy (1985) found from 10 males that at 22–28 ° C call duration averaged 4.02 s (range 1.7–6.7), segment duration averaged 100.1 ms (92–125), and segment repetition rate averaged 3.67 segments/s (2.9–4.2).
However, segment structure conspicuously and consistently differs among the three species ( Figure 5 View Figure 5 ). In H. heinzsteinitzi (in both projects) each segment had its energy peak near its temporal beginning, whereas in H. savignyi the segment’s rise and fall times are similar, energy being high throughout its middle, and in H. arborea segment energy gradually rises to peak near the segment’s end ( Figure 5 View Figure 5 ). The latter applies also to H. sarda ( Schneider 1977) while the segment shape of H. meridionalis is intermediate between those of H. arborea and H. savignyi ( Schneider 1977) .
Field notes
The type locality is interesting historically and ecologically. The Mamilla reservoir is a 97×65× 6 m (deepest point) cistern excavated in limestone and faced with limestone masonry ( Figure 6 View Figure 6 ). Allegedly dating back to the Roman period, second to third century A.D. ( Martens & Jelden 1992), it is documented (Arabic: Mamala) from the year 614 A.D. ( Tsafrir and Safrai 1999, pp. 255, 236, 340). Dry in summer, it is annually filled by winter rains to varying extent, modulated by its sloping bottom. In recent decades its newly elevated rim prevents its filling by direct rain runoff; water enters from the soaked earth through crevices in the walls ( Ortal 1997). Its history and ecology are briefly reviewed in Martens and Jelden (1992).
Translated excerpts from the collector’s notes ( Shy 1978): ‘‘Ein Fara in the Judaean desert is a spring-fed pool ( Figure 7 View Figure 7 ). The ample vegetation is dominated by Typha australis . In October 1976, on several occasions, tree frogs were sitting adpressed on the Typha stalks, vertically, head up, during 0900–1100 h and again around 1600 h. Their green colour with the dark flank merged well with the cattail. In earlier morning hours and in mid-day hours I saw none but due to their camouflage it is not sure that they were absent’’.
‘‘The Wadi at Moza: in April 1977 water depth exceeded 1 m and the vegetation, ample and tangled, was dominated by Rubus sanctus . Tree frogs sat on this spiny bush. During 0900–1200 h, visual detection being difficult, I stimulated males to call by playing tree frog calls recorded in nature (Jerusalem), in 1970, at 19 ° C. Two males answered and one was caught, its colour green. The same day around 1700 h, no males answered the played calls. But about 30 min after sunset a few calls were heard and gradually a large chorus assembled. To catch a male I would enter the water, deliver the stimulus call, approach the source of the answer in darkness, dazzle the male with torchlight, and catch it calling. All seven males caught were sitting on bush leaves close to the water, facing it. During call emission they erected the forelimbs, contracted the body, and inflated the vocal sac; pausing, they contracted the vocal sac and inflated the lungs’’.
Distribution and conservation
So far H. heinzsteinitzi is documented only from the three Judaean Hills localities from which the type series originated, within a west–east stretch of 13 km and a north–south extent of 6 km ( Figure 8 View Figure 8 ). The Mamilla reservoir ( IG 1710 1317) and the Wadi near Moza ( IG 1658 1353) lie within the Mediterranean region (sensu stricto) with average annual precipitation of 500–700 mm, and average annual temperatures of 17–19 ° C. The third locality, Ein Fara ( IG 1789 1378), is an oasis-like spring habitat on the fringe of the Judean Desert, with average annual precipitation fluctuating widely around 300 mm, and average annual temperatures around 19–21 ° C. In this area there is a steep transition between the mesic and desert zones, as travelling from Jerusalem east towards the Dead Sea, over an arial distance of 25 km, one passes through belts representing the Mediterranean, Irano-Turanian, Saharo-Arabian and Sudanian Regions (maps in Werner 1987, 1988).
Apparently Hyla heinzsteinitzi View in CoL is sympatric with H. savignyi View in CoL and in some places the two are apparently syntopic. Hyla savignyi View in CoL ranges widely from Turkey, Transcaucasia and northwestern Iran over Syria and Lebanon to central Jordan and Israel and the southwestern Arabian Peninsula (Balletto et al. 1985; Baran and Atatür 1998; Kuzmin 1999; Tarkhnishvili and Gokhelashvili 1999; Disi et al. 2001); it occurs marginally in northern Sinai (Adel Ibrahim, unpublished; Saleh 1997).
In Israel the distribution of amphibians was mapped by Mendelssohn and Steinitz (1944), Wahrman (1956) and Gerchman and U. Werner (1990). Because these authors could not have distinguished the two species of Hyla View in CoL , a map of H. savignyi View in CoL locality records is presented here besides that for H. heinzsteinitzi View in CoL .
The tiny range of H. heinzsteinitzi and its possible constraint to isolated sites significantly endanger it. Unfortunately, this danger seems aggravated by the lack of interest of the national conservation authority.
Etymology
The species is named for the late Heinz Steinitz, 26 April 1909 – 28 April 1971, Professor and Curator of Fishes at the Hebrew University of Jerusalem, in recognition of his contribution to the knowledge, and thus conservation, of the amphibians of Israel ( Clark 1971) .
| IG |
Institute of Geology |
| V |
Royal British Columbia Museum - Herbarium |
| IOM |
Institute of Oceanology, Academy of Sciences |
| ET |
East Texas State University |
| FM |
Department of Nature, Fujian Province Museum |
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hyla heinzsteinitzi
| Grach, Constantin, Plesser, Yeshurun & Werner, Yehudah L. 2007 |
Hyla arborea (Linnaeus, 1758)
| Fishelson L 1979: 327 |
| Lulav S 1978: 431 |
Hyla arborea savignyi
| Mendelssohn H & Steinitz H 1944: 294 |
| Bodenheimer FS 1937: 73 |
Hyla arborea var. savignyi
| Boulenger GA 1882: 380 |