Geoplana ficki, Amaral, Silvana Vargas Do, Oliveira, Simone Machado De & Leal-Zanchet, Ana Maria, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.281355 |
|
DOI |
https://doi.org/10.5281/zenodo.6167354 |
|
persistent identifier |
https://treatment.plazi.org/id/03E4307E-A656-FFE4-68EA-FE47FC6AFD07 |
|
treatment provided by |
Plazi |
|
scientific name |
Geoplana ficki |
| status |
sp. nov. |
Geoplana ficki sp. nov. Amaral & Leal-Zanchet
Geoplana sp. 8: Leal-Zanchet & Carbayo, 2000
Geoplana sp. 6: Fick, Leal-Zanchet & Vieira, 2006
Geoplana sp. 6: Baptista, Fick, Matos & Leal-Zanchet, 2006
Etymology. the specific name is in honor of MSc. Israel Alberto Fick and his collaboration in collecting various specimens of land planarians which were deposited in the scientific collection of the Instituto de Pesquisas de Planárias (UNISINOS).
Type material. Holotype: MZUSP PL.01146: I. A. Fick, leg. 12.VIII.1999, São Francisco de Paula, RS, Bra-zil—anterior tip: transverse sections on 151 slides; anterior region at the level of the ovaries: sagittal sections on 175 slides; pre-pharyngeal region 1: transverse sections on 48 slides; pre-pharyngeal region 2: transverse sections on 127 slides; pharynx: sagittal sections on 212 slides; copulatory apparatus: sagittal sections on 60 slides.
Paratypes: MZU PL.00101: M. Cardoso, leg. 03.IV.1998, São Francisco de Paula, RS, Brazil—anterior tip: transverse sections on 60 slides; anterior region at the level of the ovaries: sagittal sections on 29 slides; posterior region to ovaries: sagittal sections on 120 slides; pre-pharyngeal region; transverse sections on 32 slides; pharynx: sagittal sections on 139 slides; copulatory apparatus: sagittal sections on 50 slides; MZU PL 00102: A. M. Leal- Zanchet, coll. 04.V.1998, São Francisco de Paula, RS, Brazil—pre-pharyngeal region; transverse sections on 29 slides pharynx: sagittal sections on 61 slides; copulatory apparatus: sagittal sections on 40 slides; MZU PL.00103: I. A. Fick, leg. 02.IX.1998, São Francisco de Paula, RS, Brazil—copulatory apparatus: horizontal sections on 117 slides; MZU PL.00104: I. A. Fick, leg. 29.XI.1999, São Francisco de Paula, RS, Brazil—preserved in ethanol 70º; MZU PL.00105: I. A. Fick, leg. 26. III. 2000: Cambará do Sul, RS, Brazil—anterior tip: sagittal sections on 106 slides; MZU PL.00106: I. A. Fick, leg. 24.X.2000, Praia Grande, SC, Brazil—anterior tip: transverse sections on 47 slides; anterior region at the level of the ovaries: sagittal sections on 23 slides; pharynx: sagittal sections on 92 slides; copulatory apparatus: sagittal sections on 71 slides; MZU PL.00107: I. A. Fick, leg. 11.XII.2000, Cambará do Sul, RS, Brazil—pre-pharyngeal region; transverse sections on 112 slides; pharynx: sagittal sections on 178 slides; copulatory apparatus: sagittal sections on 244 slides; MZU PL.00108: I. A. Fick, leg. 11.XII.2000, Cambará do Sul, RS, Brazil—preserved in ethanol 70º; MZU PL.00110: A. M. Leal-Zanchet, coll. 06.VIII.2002, São Francisco de Paula, RS, Brazil—preserved in ethanol 70º; MZU PL.00111: P. K. Boll, leg. 24.II.2010, São Francisco de Paula, RS, Brazil—anterior tip: transverse sections on 68 slides; anterior region at the level of the ovaries: transverse sections on 82 slides; posterior region to ovaries: transverse sections on 117 slides; copulatory apparatus: horizontal sections on 185 slides.
Type locality. São Francisco de Paula, state of Rio Grande do Sul (RS), Brazil.
Distribution. Santa Catarina (Praia Grande) and Rio Grande do Sul (Cambará do Sul, São Francisco de Paula), Brazil.
Diagnosis. body broad and flat, anterior end pointed, posterior end gradually narrowed. Live specimens with dorsum brown or greenish-brown to the naked eye and venter orange to light brown with dark contour. Eyes dorsal, with inconspicuous clear halos; sensory pits in the first third of the body; conspicuous glandular margin with three types of secretory cells; mc:h, 8%–11%; pharynx collar-form; very short esophagus; esophagus: pharynx ratio, 2%–7%; anteriormost testes anterior to ovaries, most posterior ones near to root of pharynx; sperm ducts open laterally into anterior end of prostatic vesicle; prostatic vesicle tubular, mainly extrabulbar, consisting of two portions, a forked and short proximal portion and an unpaired distal portion which loops dorsally and posteriorly, entering the bulbar muscular coat; male atrium short, with the entire cavity occupied by the conical penis papilla which extends into the female atrium; straight ejaculatory duct opens through the tip of the almost symmetrical papilla; ovovitelline ducts emerging, dorsally and laterally displaced, from posterior third of ovaries, and ascending behind gonopore; common glandular ovovitelline duct short; vagina as a dorso-anteriorly curved ental portion of female atrium; female atrium elongate, with ample lumen; male atrium shorter than female atrium; length of female atrium, 123% that of male one; straight gonopore canal; no folds separating male and female atria.
Description. External morphology: Body broad and flat, anterior end pointed, posterior end gradually narrowed ( Figs. 1–2 View FIGURES 1 – 2 ). When crawling, maximum length reaches 231mm (Table 1). Mouth distance from anterior tip varies from 52% to 77%; gonopore between 70% and 88%, relative to body length (Table 1).
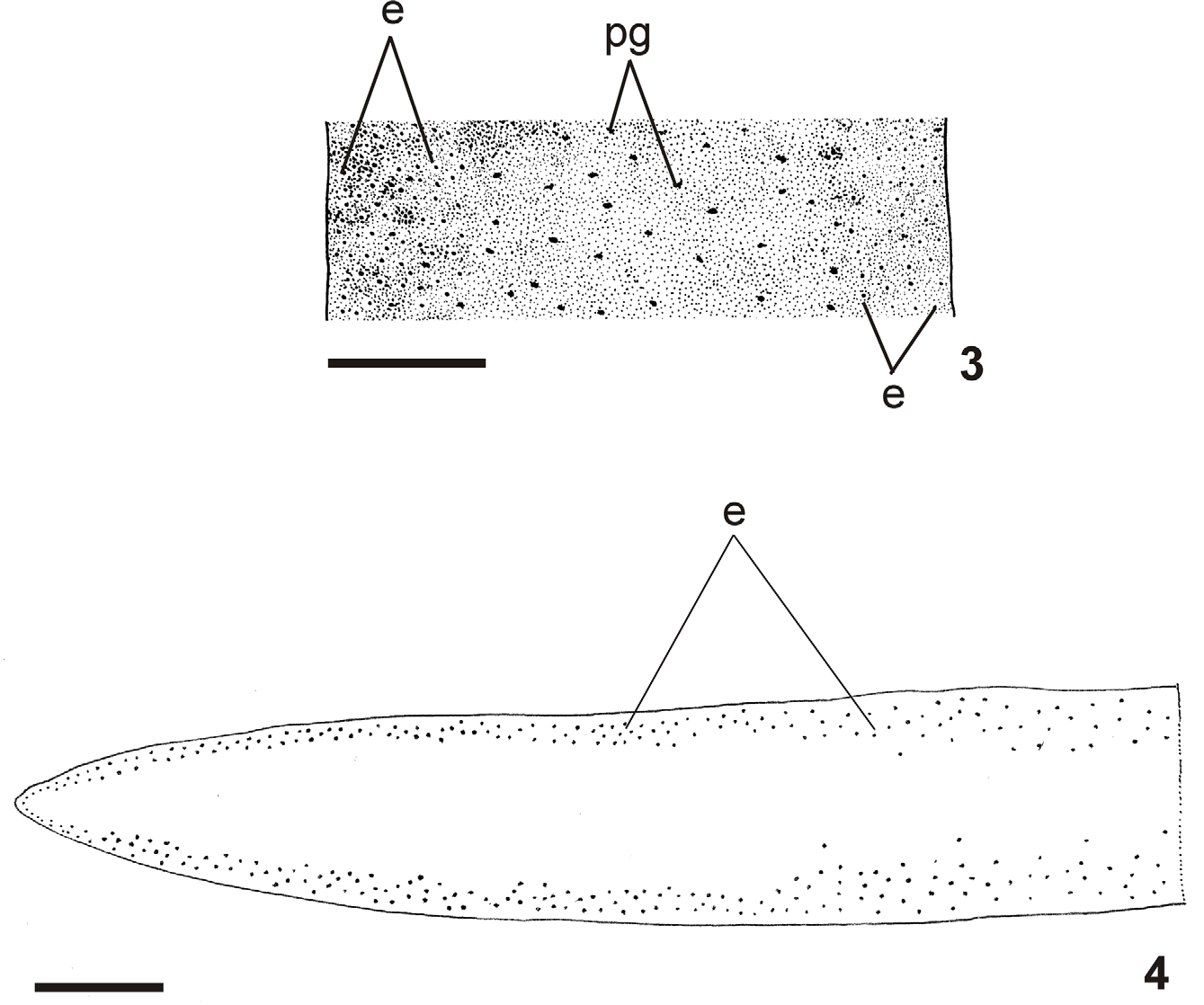
Live specimens with brown dorsum, sometimes greenish-brown, usually seeming homogeneous to the naked eye. Some specimens with pale greenish-gray dorsum, with numerous fine black spots under moderate magnification ( Fig. 1 – 3 View FIGURES 1 – 2 View FIGURES 3 – 4 ). In preserved specimens dorsal colour fades, becoming light brown. Under the stereomicroscope, dorsal ground-colour pale brown or greenish gray covered by dense dark-brown or black pigmentation. Venter orange to light brown with dark contour, becoming grayish or pale brown in preserved specimens.
Eyes uniserially surround anterior tip, becoming large and irregular in distribution immediately after the tip. Approximately after 0.5 mm and 1 mm from the tip (about 1% and 2% of body length), respectively, they form two and three series. After 3 mm or 4 mm (about 5% or 7% of body length), they also extend slightly dorsad and are surrounded by inconspicuous halos, being more abundant about 15 mm from the tip in paratype MZU PL.00110 (ca. 25% of body length). They occupy the maximum width of 3 mm on each side of the body (about 33% of body width in paratype MZU PL.00110) ( Fig. 4 View FIGURES 3 – 4 ). Towards posterior end, they become less numerous.
Internal morphology. Anterior region: Sensory pits, as simple invaginations, about 35 µm to 65 µm deep, contouring anterior tip, occurring initially at intervals of approximately 25 µm, posteriorly become gradually sparser until they disappear approximately 30 mm from anterior tip (30% of body length in paratype MZU PL.00111). Eyes contour the anterior tip in a single row, then run along both sides of the body (two to three eyes on each side). Eyes 28 µm to 48 µm diameter. Cutaneous musculature arrangement similar to that of pre-pharyngeal region (see next section), but thinner close to the tip; longitudinal fibers absent or very weak on first 0.4 mm of body length. Mesenchymal musculature, poorly developed on anterior tip, mainly comprising crossed fibers between ventral cutaneous musculature and margins of body; transverse sub-intestinal layer, together with some transverse supraintestinal and dorsoventral fibers appear about 0.4 mm after anterior tip. Rhabditogen cells absent on first 260 µm of body length. Ventral epidermis receives weakly stained cyanophil cells with granular secretion, abundant erythrophil cells with granular secretion, and numerous xanthophil cells with granular secretion. Dorsal epidermis receives weakly stained cyanophil cells with amorphous secretion and sparse rhabditogen cells on first 1.1 mm of body length (1% of body length in paratype MZU PL.00111). Secretory cells absent on sensorial border, except cyanophil cells. Xanthophil and erythrophil cells become more numerous on body margins, forming the glandular margin, approximately 0.4 mm from tip.
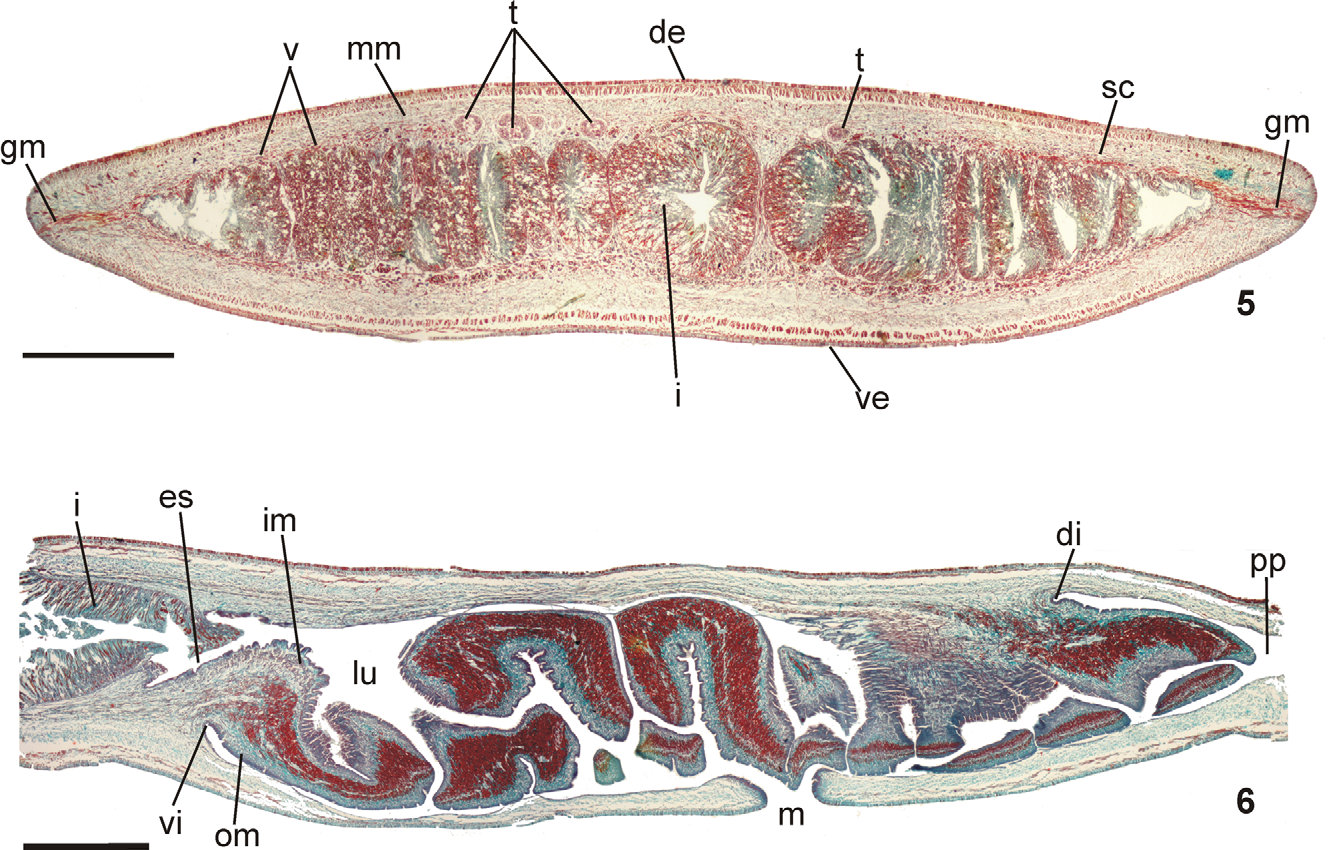
Epidermis and musculature at pre-pharyngeal region ( Fig. 5 View FIGURES 5 – 6 ):Creeping sole broad, occupying 82% to 90% of body width (Table 1). Three types of secretory cells open through dorsal epidermis and body margins: (1) numerous rhabditogen cells with xanthophil secretion and less frequent (2) weakly cyanophil cells with fine granular secretion and (3) erythrophil cells with fine granular secretion. Creeping sole receives abundant cells with weakly cyanophil amorphous secretion, rhabditogen cells, and erythrophil cells with fine granular secretion, less numerous. Glandular margin conspicuous, comprising numerous cells with coarse granular erythrophil secretion and cells with coarse granular xanthophil secretion; cells with coarse granular cyanophil secretion, cells with fine erythrophil secretion, as well as cells with mixed secretion (periphery cyanophil with a central erythrophil core) less numerous.
Cutaneous musculature tripartite (circular, oblique and longitudinal muscle layers), longitudinal layer with thick bundles. Mc:h 8% to 11% ( Table 2 View TABLE 2 ).
Mesenchymal musculature comprised mainly of transverse, oblique, and dorsoventral fibres. Supra-intestinal and sub-intestinal muscle layers, each about 5–6 fibres thick, comprised of transverse muscle fibres. Longitudinal fibers are indiscernible.
Pharynx ( Fig. 6 View FIGURES 5 – 6 ): Pharynx collar-form. Mouth located in mid-third of pharyngeal pouch. Esophagus very short, lined with ciliated columnar epithelium presenting some insunk nuclei and cyanophil secretory cells with cell bodies in mesenchyme; with muscularis of interwoven circular and longitudinal fibers. Esophagus: pharynx ratio, 2%–7%. Pharyngeal glands with cell bodies located in mesenchyme, mainly anteriorly to pharyngeal pouch. Three secretory cell types: (1) cells with fine granular erythrophil secretion (1 μm); (2) cells with cyanophil amorphous secretion, and (3) less numerous cells with fine granular xanthophil secretion. Pharyngeal outer musculature (ca. 65 µm thick) comprised of thin subepithelial layer of longitudinal muscles, followed by a thicker circular one, mixed internally with few longitudinal fibers. Circular and longitudinal muscle layers become very thin towards pharyngeal margins. Inner pharyngeal musculature (ca. 110 µm thick) comprises a thick circular subepithelial layer, mixed with some longitudinal fibers. Inner musculature gradually thins down towards pharyngeal tip.
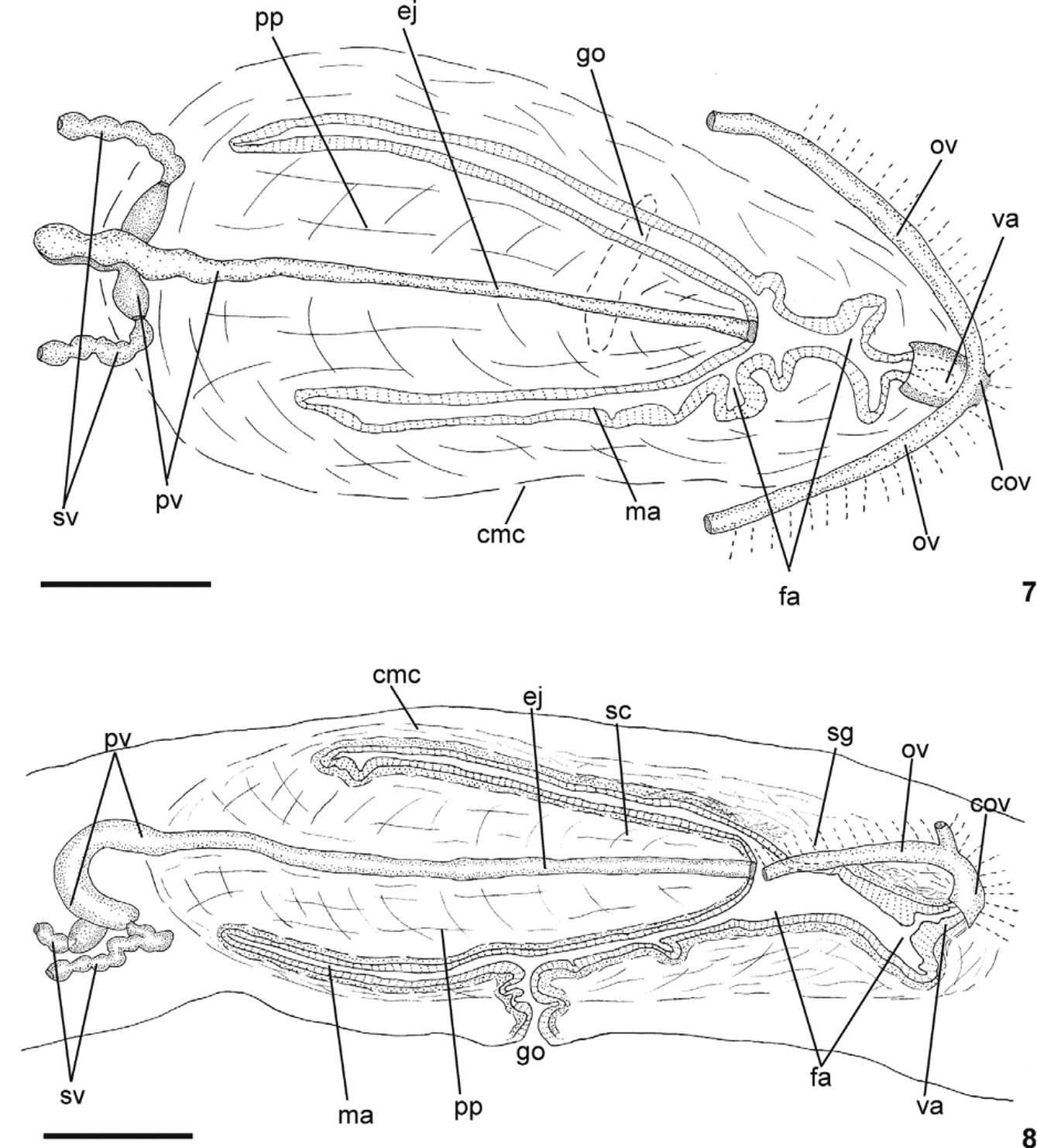
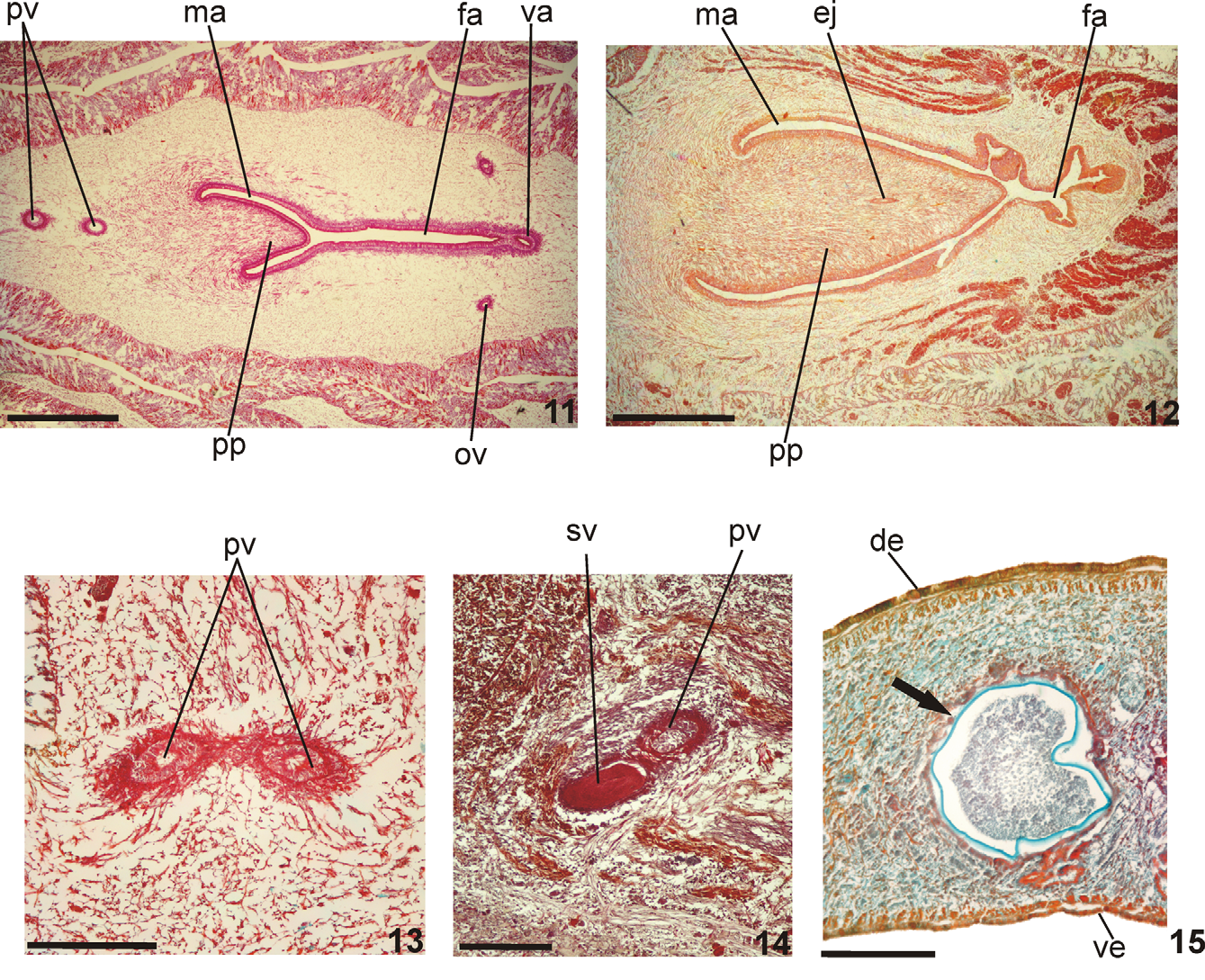
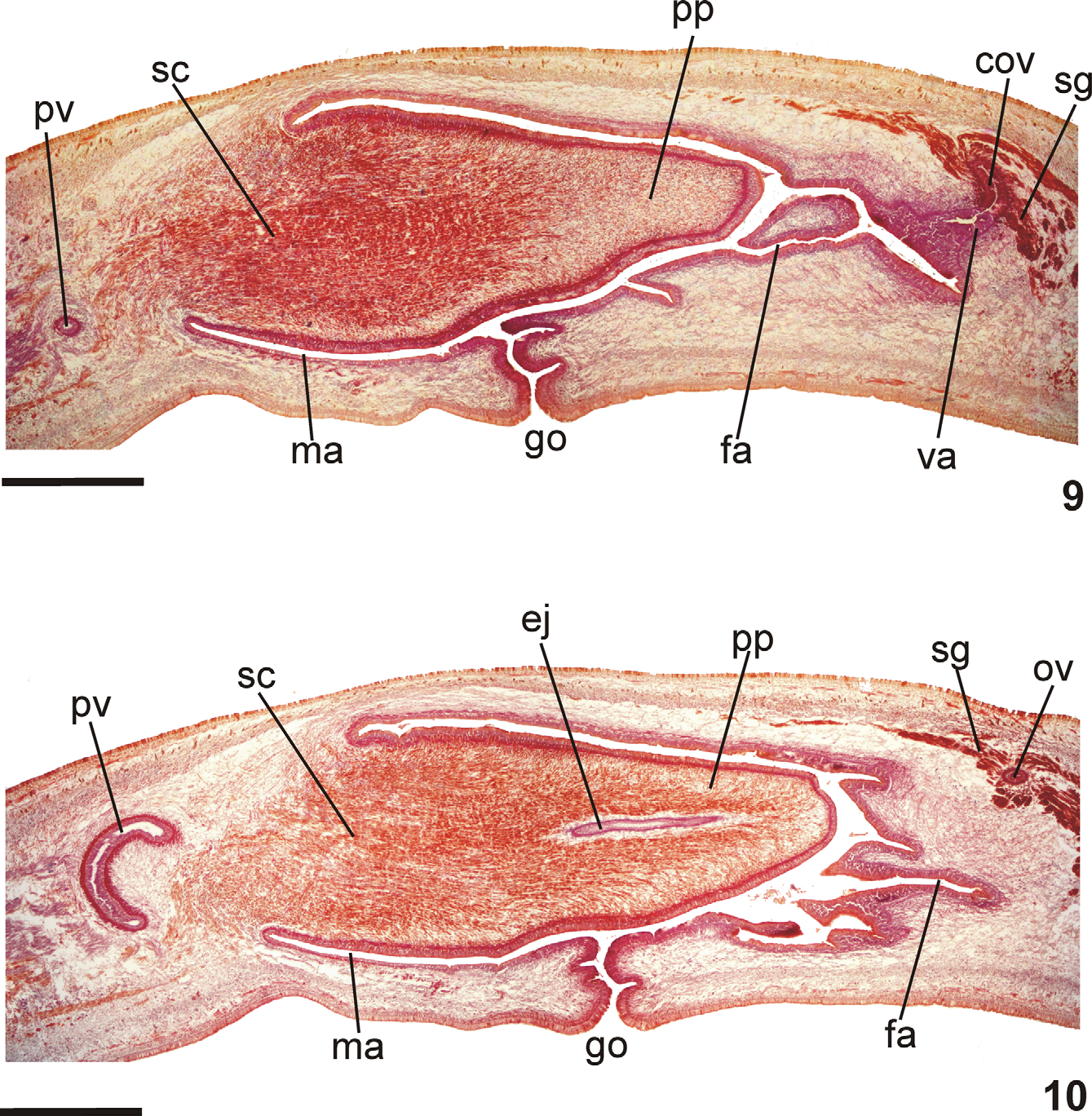
Reproductive apparatus: Testes in two irregular rows dorsal to the intestinal branches, on each side of the body extend from anterior of the ovaries to just anterior of the pharynx (Table 1, Fig. 5 View FIGURES 5 – 6 ). Pre-pharyngeally, sperm ducts, subdivided in two or three ductules, dorsal to ovovitelline ducts, medianlly displaced. Lateral to pharynx these form spermiducal vesicles, opening laterally into anterior end of prostatic vesicle ( Figs. 7, 8 View FIGURES 7 – 8 , 14 View FIGURES 11 – 15 ).
Prostatic vesicle of two portions: a short, tubular and forked proximal portion, and an unpaired distal portion which loops dorsally and then posteriorly, then enters the bulbar muscular coat and continues into the ejaculatory duct ( Figs. 11, 13, 14 View FIGURES 11 – 15 ). Ejaculatory duct traverses the long, almost symmetrical penis papilla to open through its tip. Male atrium oval-elongate, with the entire cavity occupied by the penis papilla which extends into female atrium (Table 1, Figs. 7–12 View FIGURES 7 – 8 View FIGURES 9 – 10 View FIGURES 11 – 15 ).
Sperm ducts lined by ciliated cuboidal epithelium, with thin muscularis (ca. 10 µm thick) comprised mainly of circular fibers. Prostatic vesicle lined with ciliated pseudostratified epithelium. Numerous weakly cyanophil cells with amorphous secretion and less abundant xanthophil cells with granular secretion, both with bodies lying in mesenchyme, mainly around vesicle, open into both portions of vesicle. Muscularis of vesicle (25–40 µm) comprised of interwoven circular and longitudinal fibers.
Ejaculatory duct lined with ciliated columnar epithelium, receiving numerous openings from secretory cells with amorphous, weakly cyanophil secretion and bodies in the surrounding mesenchyme. Muscularis (ca. 10 µm thick) comprised mainly of circular fibers. Penis papilla lined with columnar, non-ciliated epithelium, diminishing very slightly in height towards its tip. Three types of secretory cells run longitudinally in papilla, with numerous openings through its lining epithelium: (1) numerous cells with fine densely arranged granular erythrophil secretion; (2) less numerous cells with granular mixed secretion (cyanophil peripheral part and erythrophil central core); and (3) cells with cyanophil amorphous secretion. Erythrophil cells with cell bodies external to common muscle coat; cells with mixed secretion and cyanophil secretion, with intrabulbar or intrapapillar cell bodies. Muscularis (50 µm) composed of a circular subepithelial layer and a longitudinal subjacent layer; thinner towards the tip of papilla. Longitudinal, radial and oblique muscle fibers cross papilla.
Epithelial lining of male atrium, columnar (30 µm), non-ciliated, becoming ciliated ventrally near to gonopore. Epithelial cells with xanthophil apical secretion, higher distally. Three types of secretory cells, with cell bodies internal to common muscle coat, empty through the epithelium: cells with cyanophil amorphous secretion, cells with granular mixed secretion (cyanophil peripheral part and erythrophil central core), and cells with fine granular xanthophil secretion. Muscularis (40 µm) comprised of circular subepithelial fibers and subjacent longitudinal fibres.
Ovaries oval-elongate with a long posterior projection, measuring 1.0 mm anterior-posteriorly and 0.15 mm dorso-ventrally in the holotype. Ovovitelline ducts emerge dorsally and laterally displaced from posterior third of ovaries, then recurve immediately dorsal to nerve plate. Behind gonopore, ovovitelline ducts ascend posteriorly and medially inclined, to unite dorsally to the ental part of female atrium (proflex dorsal approach), thus forming a short common glandular ovovitelline duct. Ental portion of female atrium presents a short, dorso-anteriorly curved diverticulum (vagina) (Table 1). Female atrium oval-elongate in shape ( Figs. 7–12 View FIGURES 7 – 8 View FIGURES 9 – 10 View FIGURES 11 – 15 ). Length of female atrium 123% of male atrium length in the holotype (Table 1).
Paired ovovitelline ducts and common oviduct lined with ciliated columnar epithelium, with muscularis comprising mainly circular fibers with some interposed longitudinal fibers. Shell glands with xanthophil secretion open into distal ascending portion of paired ovovitelline ducts and common glandular ovovitelline duct.
Female atrium lined with very thick epithelium, with multilayered aspect, irregular in height ( Figs. 9, 10 View FIGURES 9 – 10 , 12 View FIGURES 11 – 15 ), up to 150 µm, reducing in height in vagina (maximum 95 µm), changing to a ciliated columnar epithelium in its ental portion. Epithelial cells of female atrium and vagina with xanthophil apical secretions. Secretions within female atrium derived from numerous cells with granular xanthophil secretion, less numerous secretory cells with cyanophil amorphous secretion, together with cells with a granular mixed secretion (cyanophil peripheral part and erythrophil central core). Epithelium of vagina receives three secretions: abundant cyanophil and mixed (peripheral cyanophil with erythrophil central core) secretions from cells external to the common muscularis, and xanthophil secretions from subepithelial glands. Muscularis of female atrium and vagina of similar thickness (20–45 µm) to that of the male atrium, comprising interwoven circular and longitudinal fibers.
Gonopore canal approximately vertical in sagittal plane. Male and female atria with ample communication, without folds separating them ( Figs. 8–10 View FIGURES 7 – 8 View FIGURES 9 – 10 ). Gonopore canal lined with ciliated columnar epithelium with numerous openings of rhabidtogen cells, cells with cyanophil amorphous secretion, and scattered cells with granular erythrophil secretion. Muscularis of circular fibers with subjacent longitudinal fibers.
Common muscle coat thin with circular, longitudinal and oblique fibers surrounding male and female atria, separated from the atrial muscularis by a poorly developed stroma with variously oriented muscle fibers.
Vitellaria, situated between intestinal branches, open into the ovovitelline ducts.
Remarks. The dorsum in paratypes MZU PL.00102 and MZU PL.00111 was olivaceous-brown covered with abundant, irregular dark-grey spots. Vitellaria, well-developed in the holotype and paratype MZU PL.00103, were, together with the shell glands, inconspicuous in paratypes MZU PL.00101, MZU PL.00102 and MZU PL.00106, thereby indicating incomplete maturity in these specimens. Although the testes in paratype MZU PL.00107 were small, the vitellaria and shell glands were well-developed. Testes were small in paratypes MZU PL.00102 and MZU PL.00106. In the former these began posterior to the ovaries, probably through incomplete development. Although the female and male atria in four specimens (MZU PL.00102, MZU PL.00103, MZU PL.00106, and MZU PL.00111) were of almost the same length, this was not so in the holotype and two other paratypes (MZU PL.00101 and MZU PL.00107, see table 1). The holotype and paratype MZU PL.00106 show pathological features ( Fig. 15 View FIGURES 11 – 15 ) represented by encysted stages of a parasite, possibly metacercariae.
TABLE 2. Cutaneous musculature and body height, in micrometer, in the median region of a transverse section of the pre-pharyngeal region, and ratio of the height of cutaneous musculature to the height of the body (mc: h index) of specimens of Geoplana ficki sp. nov.
| Holotype | Paratype MZU PL.00101 | Paratype MZU PL.00102 | Paratype MZU PL.00106 | Paratype MZU PL.00107 | |
|---|---|---|---|---|---|
| Dorsal musculature | 92 | 77 | 66 | 82 | 62 |
| Ventral musculature | 100 | 88 | 68 | 76 | 63 |
| Body height | 2495 | 1593 | 1718 | 1390 | 1543 |
| Mc:h (%) | 8 | 10 | 8 | 11 | 8 |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Continenticola |
|
Family |
|
|
SubFamily |
Geoplaninae |
|
Genus |