Spartina, Schreb.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2020.112312 |
|
DOI |
https://doi.org/10.5281/zenodo.8302678 |
|
persistent identifier |
https://treatment.plazi.org/id/03E487FB-FFCC-FF95-4175-FB5BFDFDFBB3 |
|
treatment provided by |
Felipe |
|
scientific name |
Spartina |
| status |
|
3.2. Biological potential of the Spartina View in CoL flavonoid content
Compared to other classes of flavonoid, little is available in the literature about the biological role of C -glycosidic flavonoids in plants. However, activities ascribed to C- glycosidic flavones include their functioning as antioxidants ( Ramarathnam et al., 1989; Kitta et al., 1992), efficient glucosidase and pectinase inhibitors ( Ravisé and Chopin, 1981), insect feeding attractants ( Kim et al., 1985), larvae feeding deterrents ( Haribal and Renwick, 1998), antimicrobial agents ( Li et al., 2013), promoters of mycorrhizal symbioses ( Akiyama et al., 2002), and UV-protective pigments ( Les and Sheridan, 1990). Cglycosidic flavones function as antibiotic agents through their subsequent conversion to the more toxic quinones ( Wiseman and Carpenter, 1995). In oats, they have been identified as a defence against plant-parasitic nematodes ( Soriano et al., 2004) and as crop protectant against weeds ( De Bertoldi et al., 2009; Kong et al., 2004). Isoschaftoside has been shown to act as a seed germination inhibitor ( Hooper et al., 2010).
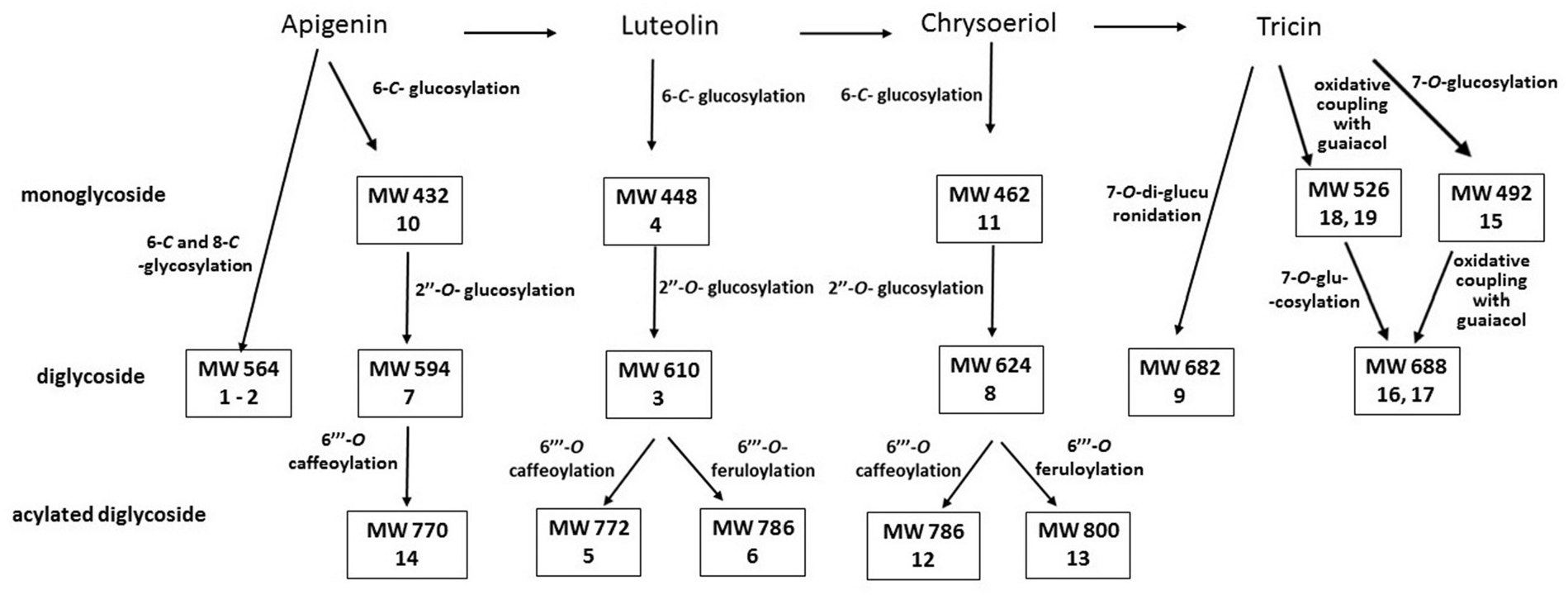
From a biosynthetic point of view, natural flavonoid glycosides are mainly synthesized by O -glycosyltransferase, but a few by C -glycosyltransferase ( Yang et al., 2018). Compared to the large accumulation of tricin-7- O -diglucuronide in S. alterniflora and S. anglica (respectively 49 and 20% of the total flavonoid, the quasi absence of O -glycosidic flavonoids in S. maritima is noticeable (1.7% of the total flavonoid).
The occurrence of tricin and tricin derivatives in plants has been reviewed (see as example Li et al., 2016). They are widely distributed in herbaceous and cereals plants, in which they exist as free tricin, tricinglycosides, tricin-lignans, and tricin-lignan-glycosides. Tricin and its derivatives were reported to function as efficient and strong antioxidants, anti-weeds, anti-herbicide and insect deterrents ( Li et al., 2016). Tricin has also been found to possess antibacterial, antifungal, insecticidal activity, and to be an effective protectant against biotic and abiotic stress ( Li et al., 2016). Strong phytotoxic activities have been reported for flavonolignans derived from tricin ( Liu et al., 2017). Tricin 7- O -glucuronopyranosyl-(2''→1‴)- O glucuronopyranoside (compound 9) has been reported to play a role in the antifeedant activity of Indian Barnyard millet extracts against brown planthopper ( Kim et al., 2008).
The reaction steps leading to tricin biosynthesis has been reconstructed as naringenin→apigenin→luteolin → chrysoeriol → selgin → tricin, and a unique flavonoid B-ring hydroxylase (CYP75B4) indispensable for tricin formation has been identified in rice ( Lam et al., 2015). This enzyme showed 5′-hydroxylase activity that was restricted to chrysoeriol, and CYP75B4 knockout mutant were found tricin deficient, with unusual accumulation of chrysoeriol ( Lam et al., 2015). Comparison of these data with our results suggests that the flavonoid 5′- hydroxylase, if present in Spartina species, should be efficient in S. alterniflora (tricin derivatives 49%; chrysoeriol derivatives 18%), but poorly expressed in S. maritima (tricin derivatives 2%; chrysoeriol derivatives 33%). From this point of view, S. anglica appears intermediate between the parental species (tricin derivatives 21%; chrysoeriol derivatives 32%) ( Table 1 View Table 1 ).
All the phenolics detected in S. anglica were found to be of parental origin, none were missing and no substitute compound was present. Of the 15 phenolics quantified, ten were inherited from S. alterniflora , three from S. maritima , and two from the two parents. The absence of substitute compounds suggests low changes of the phenolic biosynthetic pathways during the hybridization. Structural relationship between the 20 flavonoids identified in this work is presented in Fig. 6 View Fig .
In contrast to the lack of phytochemical study, the Spartina genus has been fairly well investigated at the genetic level. S. anglica results from hybridization of the genomes of S. alterniflora (maternal donor) and S. maritima (paternal donor) ( Ferris et al., 1997; Baumel et al., 2003). Hybridization is often associated with changes in biochemistry. Qualitatively, hybrids may express all of the specialized chemicals of the parental taxa, may fail to express certain parental chemicals, or may express chemicals that are absent in each parent ( Orians, 2000). The presence of all the parental phenolics in S. anglica is consistent with the parental genome additivity reported for this species ( Baumel et al., 2001, 2002). The gene expression variation between S. anlica and the two parental species has been recently reported ( Ferreira de Carvalho et al., 2017). Interestingly, a significant maternal dominance was found in S. anglica for the cinnamoyl-CoA reductase, which catalyzes the first specific step in the phenylpropanoids metabolic pathway. Its level of expression in S. anglica was found to be about the same as in S. alterniflora , and twice as high as in S. maritima ( Ferreira de Carvalho et al., 2017) . These data are in good agreement with the trend observed here with the respective phenolic contents.
Exotic plants may become invasive when they produce chemical defences that are not found in native plants. It has been shown that successful exotic plant species had a higher diversity of metabolites compared with congeneric native species ( Macel et al., 2014). Our results show that the introduced S. alterniflora has a higher diversity of phenolic than the European native S. maritima , and that the phenolic pattern of S. anglica is mostly inherited from S. alterniflora . This suggests that the phenolic chemistry of S. anglica might play a role in its invasive character. Noteworthy, S. anglica and S. maritima having been collected at the same time from two adjacent meadows only separated by a few meters, no environmental effect may be invoked to explain the difference in the phenolic content observed in this study.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |