Clavelina ossipandae, Hasegawa & Kajihara, 2024
|
publication ID |
https://doi.org/10.12782/specdiv.29.53 |
|
publication LSID |
lsid:zoobank.org:pub:E80C1459-4832-4734-8AC5-C30115A9B71C |
|
persistent identifier |
https://treatment.plazi.org/id/A0C56673-0064-4F21-BE9A-688C990FF1CE |
|
taxon LSID |
lsid:zoobank.org:act:A0C56673-0064-4F21-BE9A-688C990FF1CE |
|
treatment provided by |
Felipe |
|
scientific name |
Clavelina ossipandae |
| status |
sp. nov. |
Clavelina ossipandae sp. nov.
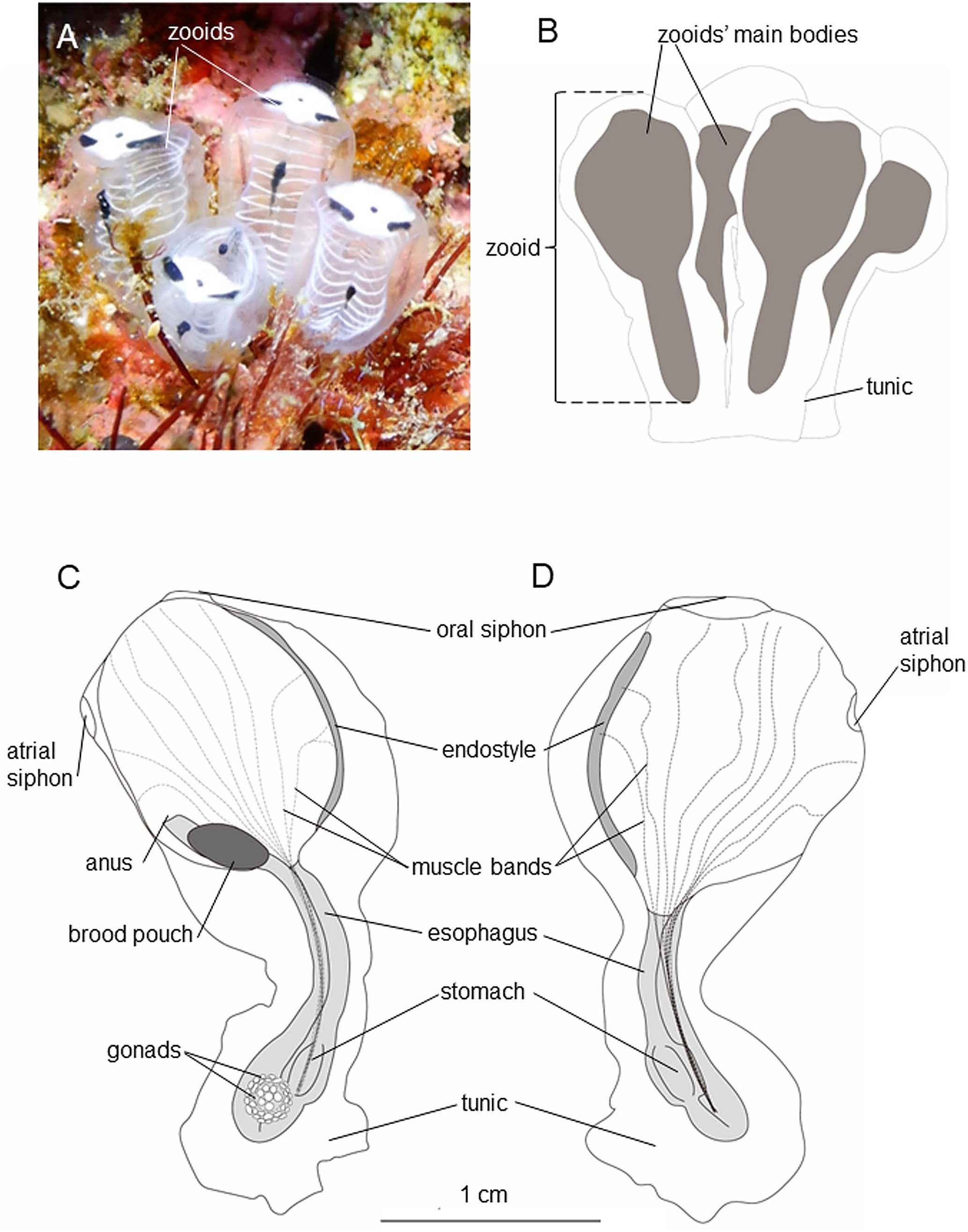
( Figs 2–4 View Fig View Fig View Fig )
Diagnosis. A Clavelina with colony consisting of zooids extending from basal mass; zooids completely free, mean 15 mm long; in life, a white, laterally elongated white patch present between oral and atrial siphons; small black point present on anterior body wall between oral and atrial siphons; an elongated black band situated laterally to the small black point on each side, slightly curved along edge of the white patch; transverse vessels white; endostyle black; short mid-dorsal black line situated posterior to atrial siphon, spanning for about four transverse vessels; 10–14 stigmatal rows in pharynx; on each side of thorax, 10 or 11 very thin longitudinal muscle bands, of which two running to endostyle, 5–6 to branchial siphon, and 2–4 to dorsal side.
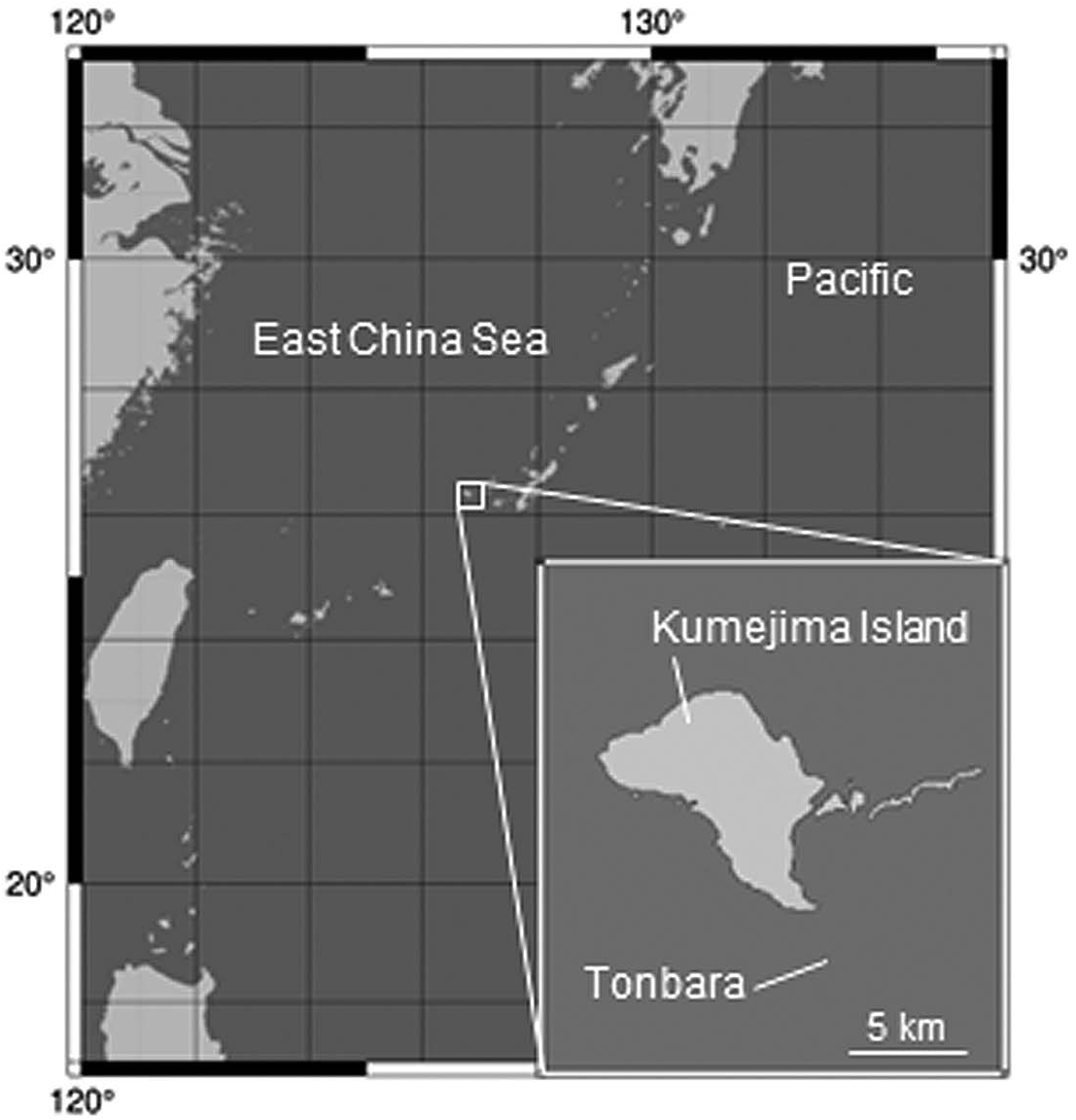
Material examined. Holotype: ICHUM 5837 View Materials , colony with four zooids, of which two had been removed for nucleotide extraction . Paratypes: ICHUM 5838–5840 View Materials , colonies with two, single, and four zooids, respectively . All were collected by N . Hasegawa, A . Izeki, and I . Nakayoshi, on 14 March 2021 at depths of 10–20 m at a diving point ( 26°16′10″N, 126°49′9″E) around a huge rock—locally called Tonbara ( Fig. 1 View Fig )—off the southeast coast of Kumejima Island in the Okinawa Islands, Japan GoogleMaps .
Description. Colony consisting of 1–4 zooids ( 4 in holotype); each zooid with its own enclosing thin tunic completely free from adjoining ones, united only by its basal tunic ( Fig. 2A, B View Fig ; Table 2). Each zooid basally connected to each other with short vascular stolon. Tunic colorless, transparent, and gelatinous; tunic of thorax softer and thinner than that of abdomen. Zooid 7–14 mm ( 14 mm in holotype, mean 9.3 mm) ( Fig. 2C, D View Fig ). In life, zooid’s main body shrinking easily upon stimulus; laterally elongated white patch present between oral and atrial siphons, width of patch almost same as that of pharynx; small black point present on anterior body wall between oral and atrial siphons; elongated black band situated laterally to the small black point on each side, positioned anterior to first stigmatal row, slightly curved along edge of white patch; transverse vessels white; endostyle black; short mid-dorsal black line situated posterior to atrial siphon, spanning for about four transverse vessels ( Fig. 2A View Fig ); after fixation in formalin, white color faded away, black point and bands remained.
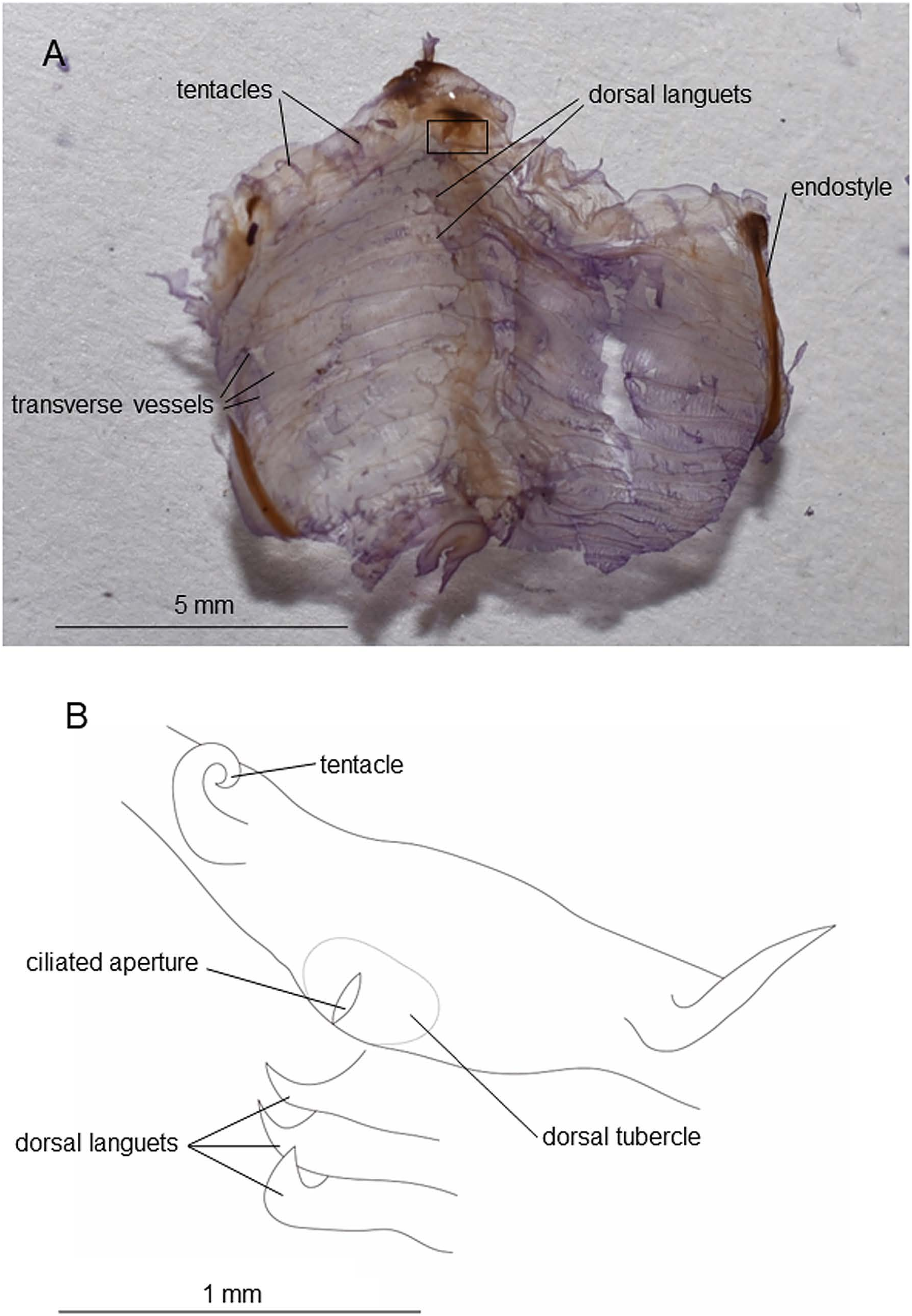
In fixed condition, thorax and abdomen almost equal in length ( Fig. 2C, D View Fig ; Table 2). Siphons having no lobes, close to each other, and smooth-rimmed; diameter of oral siphon twice larger than atrial siphon ( Fig. 2C, D View Fig ). Ten oral tentacles, each approximately 0.5 mm in length ( Fig. 3A, B View Fig ). Longitudinal muscle bands 10 or 11 ( 11 in holotype) in number, running on each side of thorax; two of them running to endostyle, 5 or 6 ( 5 in holotype) to branchial siphon, 2–4 to dorsal side ( 4 in holotype) ( Fig. 2B, C View Fig ; Table 2). Peripharyngeal groove made up of single lamina. Ciliated aperture of dorsal tubercle forming longitudinal slit ( Fig. 3B View Fig ). Pharynx without folds, longitudinal vessels, and papillae. Stigmatal rows 10–14 in number ( 14 in holotype) ( Table 2). Approximately 50 stigmata contained in each half-row. Dorsal languets present ( Fig. 3A, B View Fig ). Brood pouch black in color, situated in dextro-dorso-posterior position of thorax ( Fig. 2B View Fig ).
Esophagus opening to posterior end of pharynx. Stomach with five longitudinal folds, positioned almost in middle of abdomen ( Fig. 2B, C View Fig ). Post-stomach indistinct. Intestine isodiametric from pylorus to anus. Anus without lobes, lying about two-thirds position from anterior end of thorax.
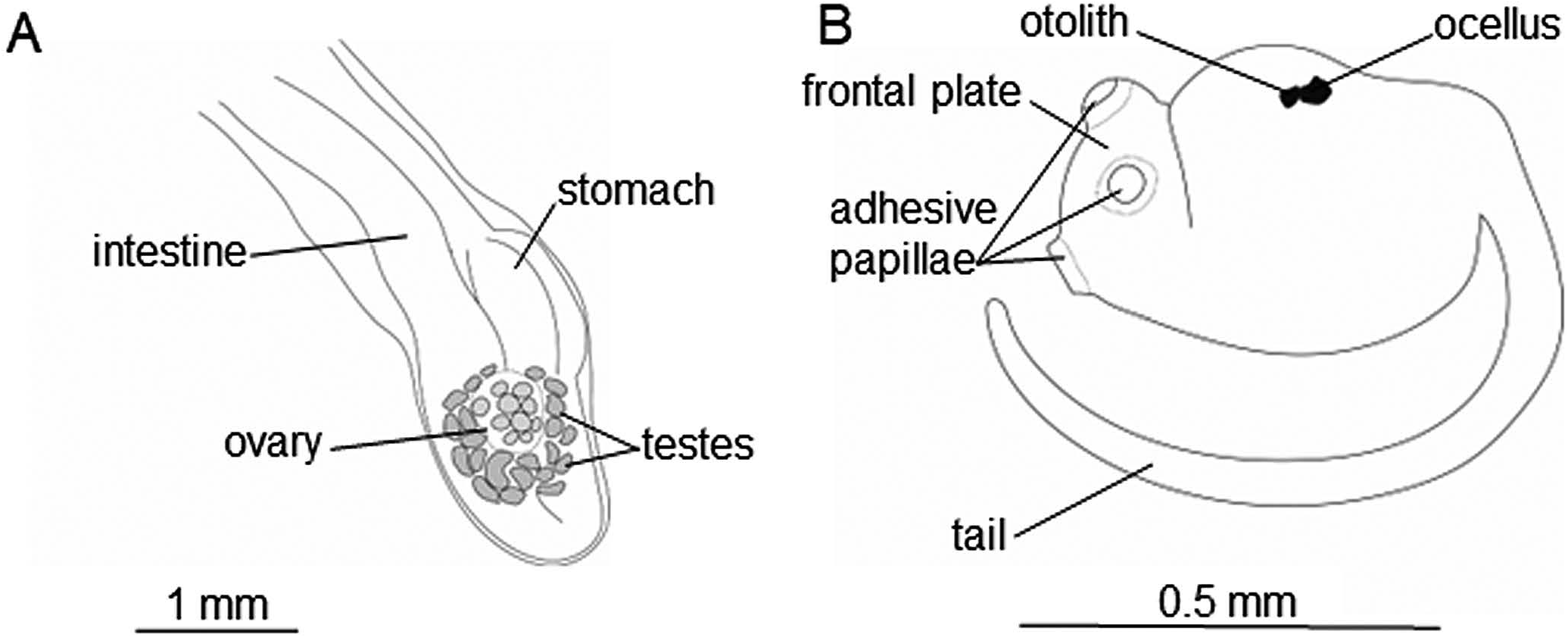
Gonads attached on left side of intestinal loop, posterior to stomach ( Figs 2C View Fig , 4A View Fig ). Ovaries surrounded with testicular follicles, latter being 0.05–0.2 mm in long-axis length, ca. 20 in number ( 22 in holotype) ( Figs 2C View Fig , 4A View Fig ). Eggs and larvae brooded in brood pouch; one larva contained in ICHUM 5837 View Materials ( holotype); 20 eggs and 12 embryos in ICHUM 5838 View Materials ( paratype) . Larvae approximately 1.25 mm in length, having ca. 0.5-mm trunk and ca. 0.75-mm tail; three protruding adhesive organs arranged in triangle on frontal plate; ocellus and otolith present in dorsal side of trunk ( Fig. 4B View Fig ).
Etymology. The new specific name, ossipandae , is a noun in the genitive case, a composite derived from os (‘bone’ in Latin) and ‘ Panda,’ the latter is meant to be the giant panda, Ailuropoda melanoleuca David, 1869, and herein treated as a noun that is latinized. The species is so named because the white anterior portion of the zooid with the characteristic black markings resembles to the face of the giant panda and the white transverse vessels evoke the ribs of a skeleton.
Distribution. Currently, the new species is only known from a diving point, Tonbara, near Kumejima Island in the East China Sea. It is difficult to access this point except in winter due to tidal currents and wind direction. The depth of this area is 10– 20 m.
COI sequence. Partial sequences (810 bp) of the COI gene were determined from the holotype ( ICHUM 5837 View Materials ) and one of the paratypes ( ICHUM 5838 View Materials ) . The K2P distance between the two specimens was 0.0126. Variations were found in 10 sites between the two: 60 (G in the holotype vs. A in the paratype), 213 (G vs. A), 225 (G vs. C), 246 (G vs. A) 340 (A vs. G), 348 ( T vs. C), 474 ( T vs. C), 477 (C vs. T), 594 (C vs. T), and 801 (A vs. G) . There was no difference when they were translated into amino-acid sequences.
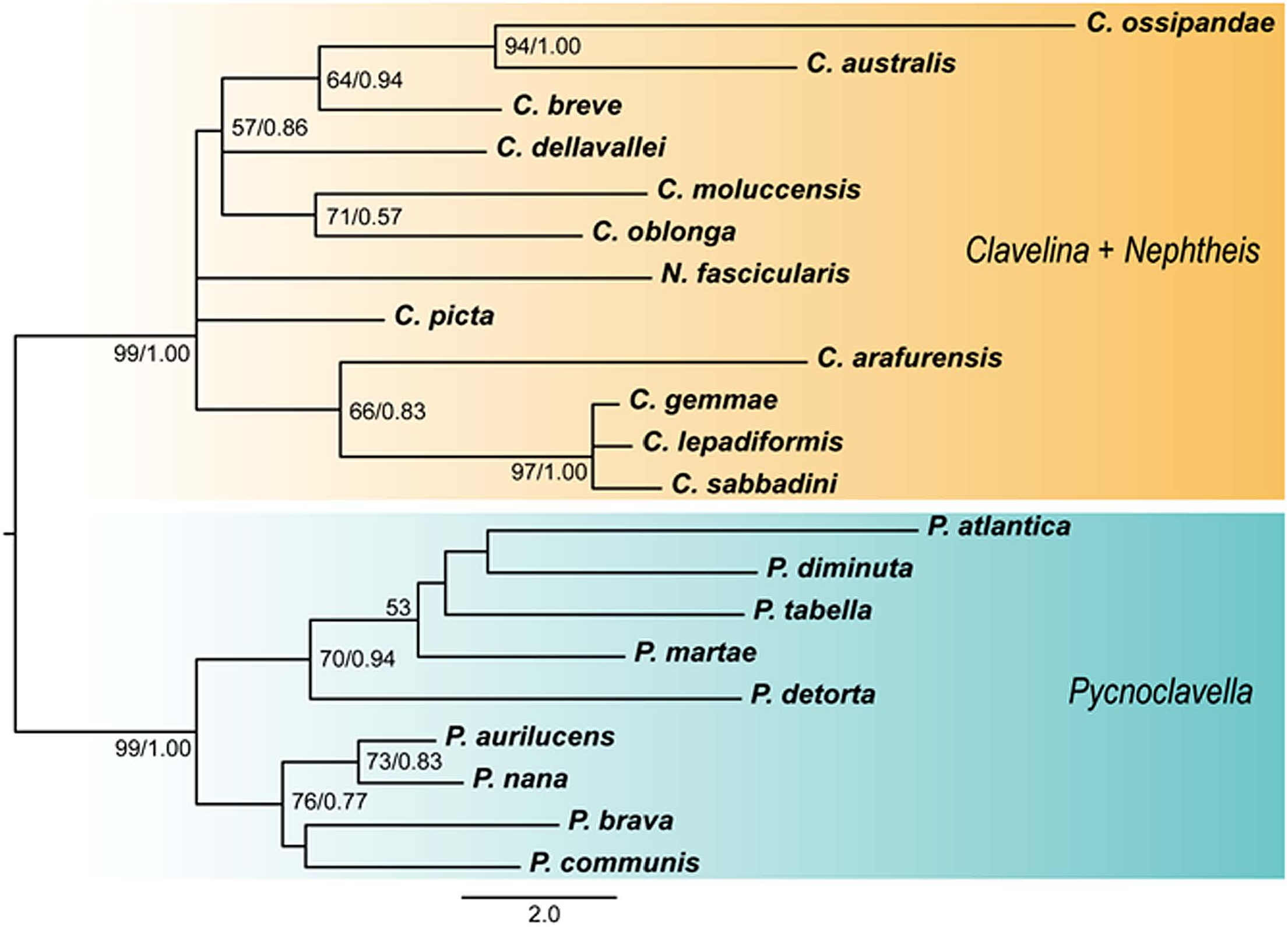
Phylogeny. Our results were similar to the phylogenetic analysis by Pérez-Portela and Turon (2008), which inferred that Clavelina and Pycnoclavella form a separate clade and that Nephtheis is in the Clavelina clade ( Fig. 5 View Fig ). In our phylogenetic tree, C. ossipandae turned out to be sister to C. australis ( Herdman, 1899) with well-supported values (UF bootstrap value, 94; posterior probability, 1.00) ( Fig. 5 View Fig ).
Remarks. The present new species can be regarded as a member of the genus Clavelina based on the following three morphological characteristics. First, the colony in C. ossipandae consists of free zooids, in contrast to the genera Euclavella and Nephtheis , where zooids are completely embedded within colonies. Second, the larvae of C. ossipandae does not possess tubular invaginated structures in the adhesive organs which is characteristic in ones of the genus Pycnoclavella ( Kott 1990; Pérez-Portela et al. 2007; Rocha et al. 2012a). Thirdly, the rows of stigmata in C. ossipandae are 10–14 in number; according to Rocha et al. (2012a), these are 8–20 in Clavelina and 3–8 in Pycnoclavella . Moreover, our phylogenetic analysis supported that this species belongs not to Pycnoclavella but to Clavelina ( Fig. 5 View Fig ).
The color pattern of C. ossipandae is unique among congeners ( Fig. 2A View Fig ; Table 1). This species is similar to C. moluccensis ( Sluiter, 1904) and C. viola in that they share a pattern consisting of a small point between the siphons and a pair of elongated bands lateral to the point (cf. Kott 1990; Ota et al. 2020). Each of the three species has a different coloration. The anterior part of the zooid is light blue in C. moluccensis , white in C. ossipandae , and yellow in C. viola ; the points and the elongated bands are dark blue in C. moluccensis , black in C. ossipandae , and blue in C. viola . The transverse vessels are blue in C. moluccensis , white in C. ossipandae , and not pigmented in C. viola . The endostyle is colorless in C. moluccensis , black in C. ossipandae , and blue in C. viola . There is no dorsal line in C. moluccensis , but it is present in C. ossipandae (black) and C. viola (blue).
Clavelina ossipandae also differs from the two congeners C. moluccensis and C. viola in the number and arrangement of muscle bands in the thorax, which are useful discriminatory traits in preserved specimens ( Tokioka and Nishikawa 1976; Kott 1990). In C. ossipandae , the number of the muscular bands running longitudinally to the endostyle is two, whereas it is six to seven in C. viola ( Tokioka and Nishikawa 1976) . In C. moluccensis, Kott (1990) reported all muscle bands are transverse based on material from Australia, whereas Sluiter (1904) did not mention anything about muscle bands in the type material from Indonesia.
The body coloration in living state is not known for the seven congeners: C. borealis Savigny, 1816 ; C. brasiliensis ( Millar, 1977) ; C. concrescens Hartmeyer, 1924 ; C. fasciculata Van Name, 1945 ; C. kottae ( Millar, 1960) ; C. michaelseni Millar, 1982 ; and C. simplex Kott, 2006 ( Table 1). However, C. ossipandae can be distinguished from all of them: by the greatest zooid length from C. borealis ( 160 mm), C. brasiliensis ( 75 mm), and C. kottae ( 120 mm) (vs. 20 mm in C. ossipandae ) ( Table 1) (cf. Savigny 1816; Hartmeyer 1903; Monniot 2001; Brunetti and Mastrototaro 2017); by the colony form from C. consrescens and C. simplex (zooids partially embedded and grouped vs. zooids completely free in C. ossipandae ) ( Table 1) (cf. Hartmeyer 1924; Van Name 1945; Kott 2006); by the number of zooid(s) from a single stalk from C. fasciculata (more than two vs. one in C. ossipandae ) (cf. Van Name 1945); and by the number of stigmatal rows from C. michaelseni (30 vs. 10–14 in C. ossipandae ) (cf. Millar 1982) ( Table 1).
Prior to our study, there had been no record of Clavelina or other ascidians from Kumejima Island according to Tokioka (1963) and Nishikawa (1995). Further research is needed to understand the ascidian fauna of this region.
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |