Archegozetes Hox
|
publication ID |
https://doi.org/10.24349/pjye-gkeo |
|
persistent identifier |
https://treatment.plazi.org/id/03E887C2-523E-FFDD-FE2D-FBC4FD27CA46 |
|
treatment provided by |
Felipe |
|
scientific name |
Archegozetes Hox |
| status |
|
The Archegozetes Hox View in CoL cluster
The Hox genes are a group of highly conserved transcription factor-encoding genes that are used to pattern the antero-posterior axis in bilaterian metazoans ( Holland and Hogan,
1988; Hrycaj and Wellik, 2016). Ancestrally, arthropods likely had ten Hox genes arranged in a cluster ( Hughes and Kaufman, 2002). During arthropod development, the Hox genes specify the identities of the body segments, and mutations in Hox genes usually result in the transformation of segmental identities ( Hughes and Kaufman, 2002). The importance of Hox genes in development of metazoans makes knowledge of their duplication and disappearances important for understanding their role in the evolution of body plans ( Hughes and Kaufman,
2002).
Mites largely lack overt, external signs of segmentation, other than the serially arranged appendages of the prosoma ( Dunlop and Lamsdell, 2017). Signs of segmentation in the posterior body tagma, the opisthosoma, do exist in adult members of Endeostigmata ( van der
Hammen, 1970). However, these segmental boundaries are largely present only in the dorsal opisthosoma, making it difficult to assess how these correspond to the ventral somites ( van der Hammen, 1970 ; Dunlop and Lamsdell, 2017). Developmental genetic studies of the spider mite and Archegozetes suggest that acariform mites only pattern two segments in the posterior body region, during embryogenesis ( Grbić et al., 2011 ; Barnett and Thomas, 2012 ; 2013b ;
2018). This stands in stark contrast to other studied chelicerate embryos. For example, during embryogenesis the spider Parasteatoda tepidariorum patterns twelve opisthosomal segments
( Schwager et al., 2015) and the opilionid Phalangium opilio patterns seven ( Sharma et al.,
2012). Furthermore, a member of Parasitiformes, the tick Rhipicephalus microplus , appears to pattern eight opisthosomal segments during embryogenesis (Santos et al., 2013).
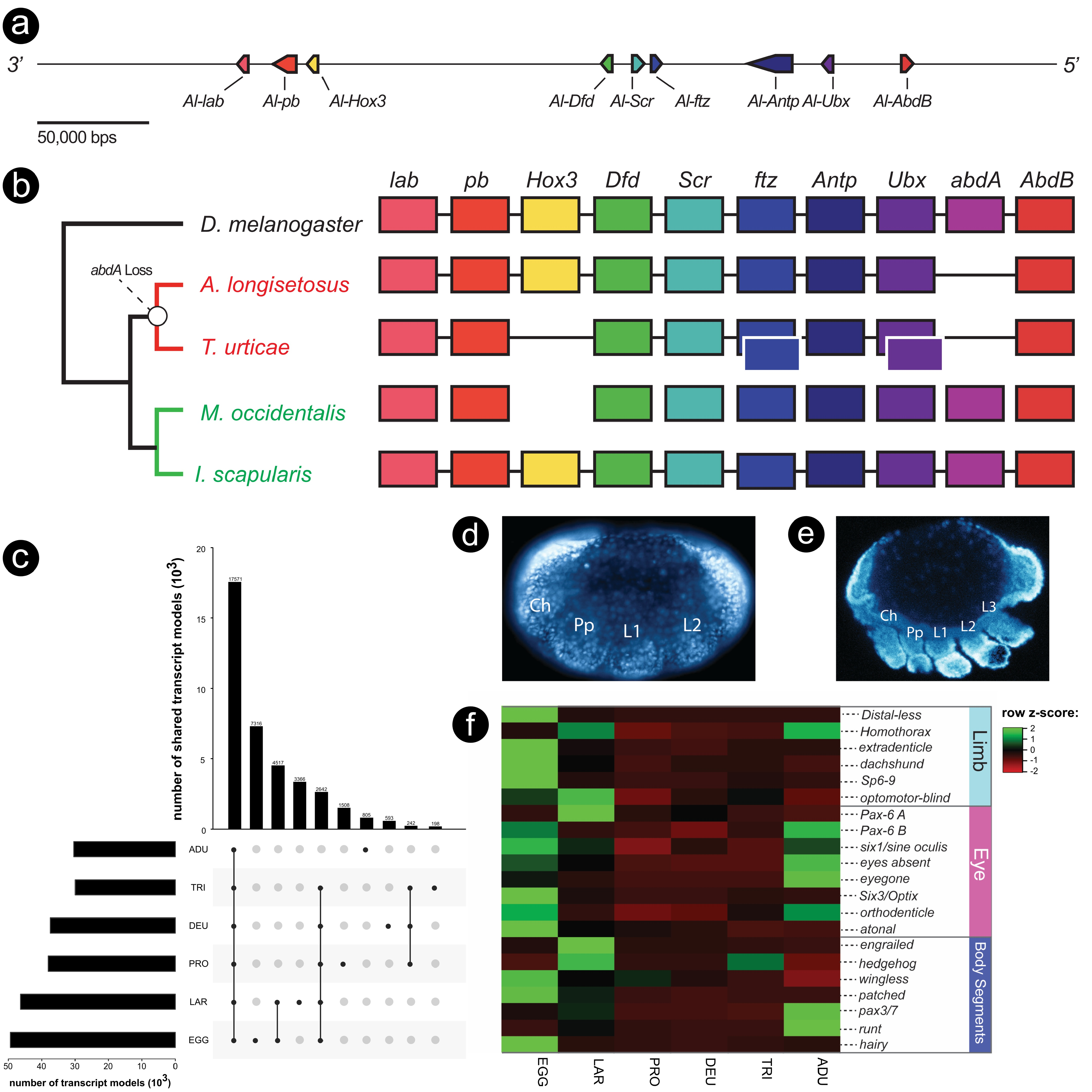
Parallel to the observation of segmental reduction in the spider mite, genomic evidence suggests that this acariform mite has lost two of its Hox genes, i.e., Hox3 and abdominal-A (abd-
A) ( Grbić et al., 2011). Interestingly, orthologs of abd-A in other studied arthropods pattern the posterior segments as well. A genomic comparison of arthropod Hox clusters has also shown a correlation between independent losses of abd-A and a reduction in posterior segmentation ( Pace et al., 2016). To investigate whether the loss of segmentation in Archegozetes is also due to an absence in abd-A, we annotated its Hox cluster, paying close attention to the region between the Hox genes Ultrabithorax ( Ubx) and Abdominal-B ( Abd-B), which is usually where this gene resides in other arthropods ( Hughes and Kaufman, 2002). Our results suggest that the Archegozetes Hox genes are clustered in a contiguous sequence (HiC scaffold 3, total size ~12.36 Mbp) in the same order as suggested for the ancestral arthropod ( Heethoff and Rall,
2015). Furthermore, we found no sequences suggestive of an abd-A ortholog in Archegozetes ( Figure 5a View Figure 5 ). These data also support the findings of a previous PCR survey that retrieved no abd-A ortholog in Archegozetes ( Cook et al., 2001) . Genomic evidence from the Parasitiformes Ixodes scapularis and Metaseiulus occidentalis reveal that these taxa maintain orthologs of all ten Hox genes, however in M. occidentalis these genes are not clustered as they are in I. scapularis ( Gulia-Nuss et al., 2016 ; Hoy et al., 2016).
Taken together, these observations suggest that the last common ancestor of acariform mites likely lost its abdominal-A gene as well as experiencied a reduction in opisthosomal segmentation ( Figure 5b View Figure 5 ). Alternatively, these shared losses of abd-A may be due to convergence due to similar selective pressures favoring a reduction in body size. The dorsal, external segmentation of endeostigmatid mites does not necessarily contradict the hypothesis of a loss of abd-A at the base of the acariform mites. As Hox genes are usually deployed after the genetic establishment of segments in arthropods ( Hughes and Kaufman, 2002), the opisthosomal segments in endeostigmatid mites may still develop in the absence of abd-A. However, this hypothesis needs further testing with observations of segmental gene expression in endeostigmatids as well as additional acariform species.
Life-stage specific RNA expression patterns
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.