Ovilyra fuliginosa ( Targioni-Tozzetti, 1877 ), 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4952.2.9 |
|
publication LSID |
lsid:zoobank.org:pub:28006273-1456-47EC-8888-17CAB686C99F |
|
DOI |
https://doi.org/10.5281/zenodo.4695030 |
|
persistent identifier |
https://treatment.plazi.org/id/03ED87AC-FF9C-FFB6-FF2D-FE2683844E01 |
|
treatment provided by |
Plazi |
|
scientific name |
Ovilyra fuliginosa ( Targioni-Tozzetti, 1877 ) |
| status |
comb. nov. |
Ovilyra fuliginosa ( Targioni-Tozzetti, 1877) View in CoL n. comb.
( Figs. 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Philyra sp. Targioni-Tozzetti 1872: 396.
Philyra fuliginosa Targioni-Tozzetti, 1877: 201 View in CoL , pl. 12 fig. 3a–g; Serène 1968: 46; Ng et al. 2008: 93; Galil 2009: 281.
Philyra olivacea Rathbun, 1909: 108 View in CoL ; Rathbun 1910: 312, fig. 4, p1. 11, fig. 17; Rathbun 1931: 99; Serène 1968: 46; Dai et al. 1986: 81, fig. 42, pl. 10(3); Dai & Yang 1991: 91, fig. 42, pl. 10(3); Tan 1995: 469, figs. 3b, d; Chen & Sun 2002: 401, fig. 181; Ng et al. 2008: 93; Galil 2009: 281.
Philyra aff. fuliginosa View in CoL — Yang 1979: 5.
Pseudophilyra olivacea — Shen & Dai 1964: 29, unnumbered fig.
Material examined. 1 male (10.4 × 12.2 mm), 1 female (8.5 × 10.1 mm) ( ZRC 2020.0345 View Materials ), station BLB, Jeram Polychaete Reef , intertidal muddy shore and intermittent sabellariid reefs, middle shore, Jeram , Kuala Selangor, West Malaysia, 3°13’27”N 101°18’13”E, coll. J. J. Eeo, 16 October 2012 GoogleMaps ; 1 male (6.9 × 8.7 mm) ( ZRC 1970.8.8.7), Muar , Johor, Peninsular Malaysia, coll. M. W. F. Tweedie, October 1938 ; 1 male (11.0 × 13.1 mm) ( ZRC 2020.0363 View Materials ), Sungei Buloh , Kuala Selangor, Selangor, Peninsular Malaysia, coll. 13 November 2011 ; 4 males (11.2 × 14.0 mm, 12.3 × 14.8 mm, 12.7 × 15.8 mm, 13.3 × 16.2 mm), 3 ovigerous females (9.3 × 11.6 mm, 9.9 × 11.8 mm, 10.4 × 12.2 mm) ( ZRC 2009.0217 View Materials ), off Changi, Singapore, coll. P. K. L. Ng, 17 April 1996 ; 1 male (6.8 × 8.0 mm) ( ZRC 2014.0176 View Materials ), Singapore River mouth, dredge, coll. Reef Ecology Study Team, 18 November 1987 ; 1 male (6.9 × 8.1 mm) ( ZRC 1990.8338 View Materials ), Kallang River basin, dredge, dredge, coll. Reef Ecology Study Team, 22 February 1989 ; 1 female (7.8 × 9.3 mm) ( ZRC 2001.2242 View Materials ), Kallang River basin, dredge, coll. Reef Ecology Study Team, 23 February 1989 .
Diagnosis. As for genus.
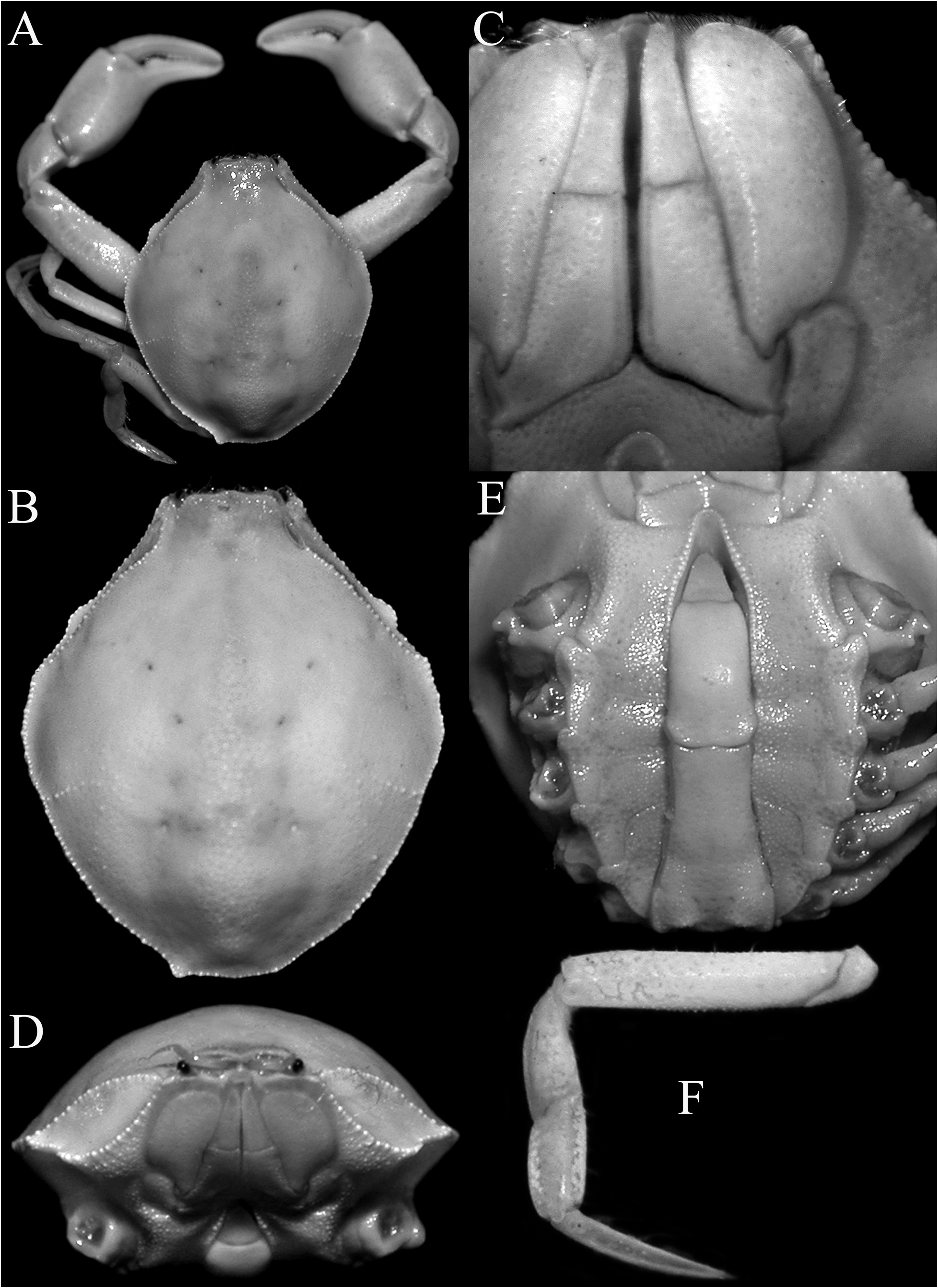
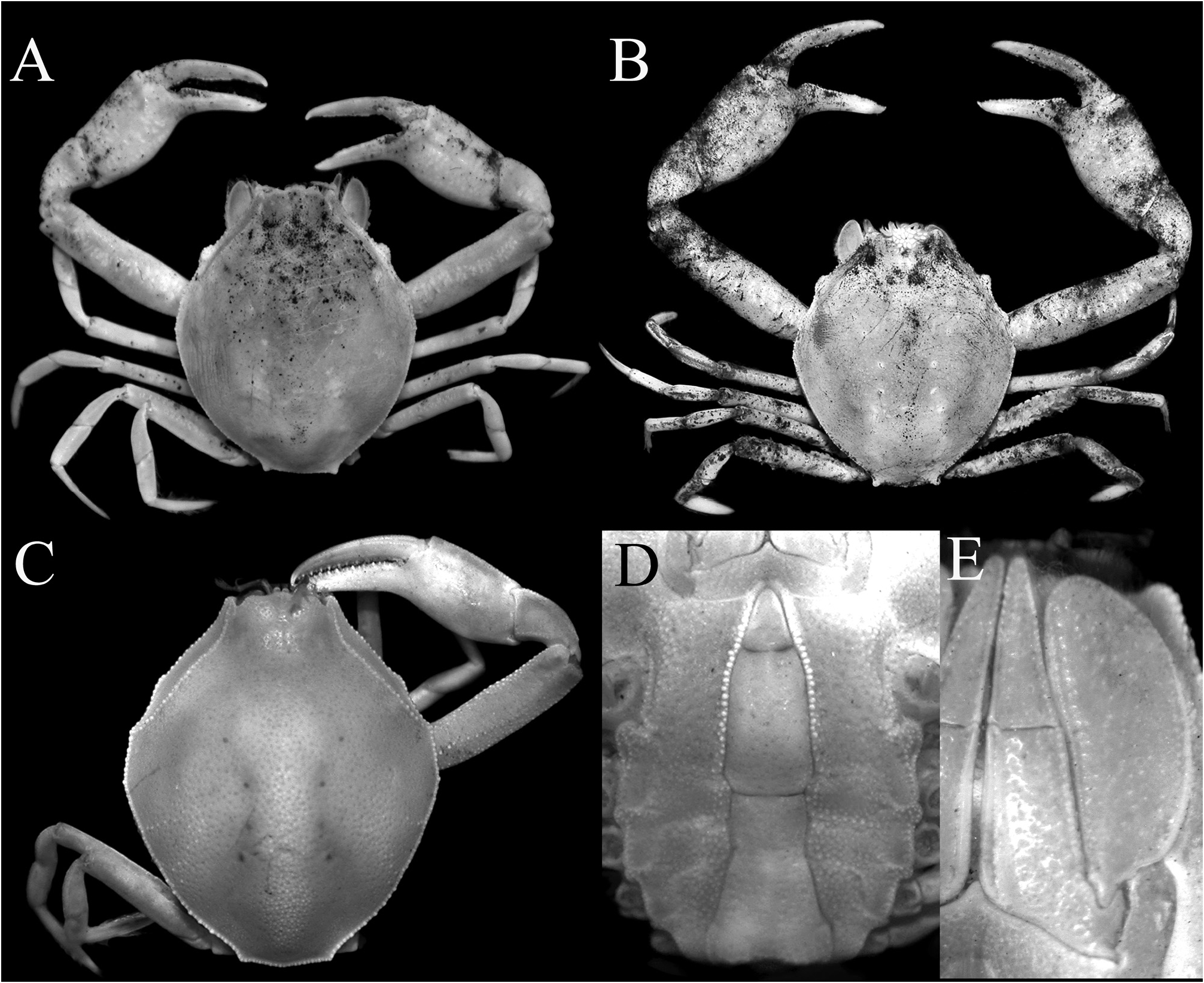
Description of male. Carapace longitudinally ovate, distinctly longer than wide (length to width ratio 1.17– 1.26); most regions indistinct, branchio-cardiac grooves just visible, cardiac region distinct, swollen; dorsal surface glabrous, covered with minute to small granules and small depressions; branchial region usually with oblique row of granules, sometimes undiscernible ( Figs. 1A, B View FIGURE 1 , 2A, B View FIGURE 2 , 3A–C View FIGURE 3 ). Frontal region not produced anteriorly, with shallow median longitudinal depression; frontal margin granulated, with low median triangular tooth ( Figs. 1B View FIGURE 1 , 2B View FIGURE 2 , 5D, E View FIGURE 5 ). Antennules folding transversely. Antennae short, longitudinally inserted between antennular fossa and base of ocular peduncle. Orbits small, rounded, upper orbital margin entire. Eyes short, retractable ( Figs. 1D View FIGURE 1 , 2D View FIGURE 2 ). Anterior margin of efferent branchial channel strongly concave, not projecting beyond frontal margin, separated from crenulate efferent margin on subhepatic region by longitudinal groove. Hepatic facet well defined by rows of granules and cristae, elongate; distal part with low granules, almost reaching frontal margin; distal part of lower margin prominently angular, with broad tooth on distal one-third ( Figs. 1B, D View FIGURE 1 , 2B, D View FIGURE 2 , 4A View FIGURE 4 ). Lateral and posterior margins of carapace irregularly granulated; posterolateral margin convex, usually demarcated from posterior margin by sharp lobiform tooth; posterior margin gently convex ( Figs. 1A, B View FIGURE 1 , 2A, B View FIGURE 2 , 3A–C View FIGURE 3 ). Pterygostomial region granulated; subbranchial region almost smooth ( Figs. 1D View FIGURE 1 , 2D View FIGURE 2 , 4A View FIGURE 4 ).
Third maxilliped exopod wider than basal part of endopod, forming petaliform structure with strongly convex outer margin, with submarginal row of low to very low granules along inner margin ( Figs. 1C View FIGURE 1 , 2C View FIGURE 2 , 3E View FIGURE 3 , 5A–C View FIGURE 5 ); endopod with completely fused basis-ischium, distinctly longer than merus, with shallow submarginal sulcus near inner margin; merus acutely triangular with pointed tip, margins unevenly granulated; palp on inner surface, dactylus longest ( Figs. 1C View FIGURE 1 , 2C View FIGURE 2 , 3E View FIGURE 3 , 5A–C View FIGURE 5 ). Coxa prominent, forming curved plate ( Figs. 1C View FIGURE 1 , 2C View FIGURE 2 , 3E View FIGURE 3 , 4A View FIGURE 4 , 5A–C View FIGURE 5 ).
Chelipeds subequal, longer, more robust in adults; merus subcylindrical, symmetrical along entire length, surface minutely granulate, especially along margins; carpus smooth, unarmed ( Figs. 1A View FIGURE 1 , 2A View FIGURE 2 , 3A–C View FIGURE 3 ). Chela stout, surfaces smooth; fingers as long as or subequal to length of palm, pollex bent at angle of 45–60º along longitudinal axis; cutting edge of pollex with broad, large subproximal tooth lined with denticles, rest of edge with small denticles; cutting edge of dactylus with subproximal concavity lined with denticles, rest of edge with small denticles ( Figs. 1A View FIGURE 1 , 2A View FIGURE 2 , 3A–C View FIGURE 3 ).
First to fifth ambulatory legs slender, short; second and third legs longest, fourth leg shortest; merus distinctly longer than carpus and propodus, margins lined with small granules (more prominent on ventral margin); dactylus longer than propodus, lanceolate, terminating in cornute tips ( Figs. 1A, F View FIGURE 1 , 2A, F View FIGURE 2 , 3A, B View FIGURE 3 ).
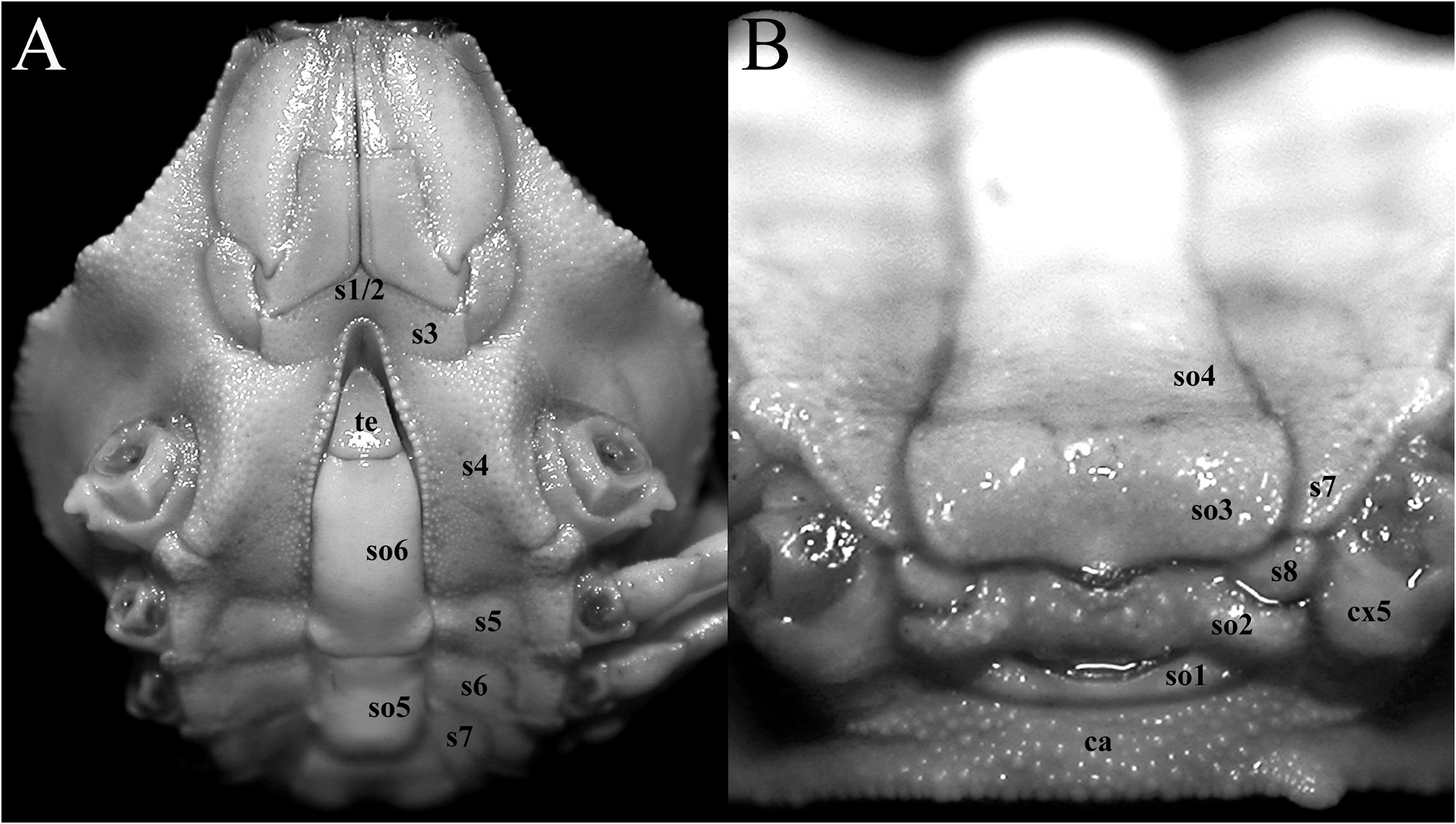
Thoracic sternites transversely narrow, surface finely granulated ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 3D View FIGURE 3 , 4A View FIGURE 4 ); sternites 1–3 completely fused without trace of sutures; sternite 3 separated from sternite 4 by shallow groove ( Fig. 4A View FIGURE 4 ); exposed sternites 4–7 progressively narrower ( Fig. 4A, B View FIGURE 4 ); sternite 8 visible when pleon closed, between margins of somites 2 and 3 ( Fig. 4B View FIGURE 4 ). Penis arising under constriction between sternites 7 and 8. Sternopleonal cavity deep, reaching to mid-distance between fused thoracic sternites 1–3 ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 4A View FIGURE 4 ); margin lined with granules, those on distal part proportionately larger ( Fig. 4A View FIGURE 4 ); pleonal locking mechanism formed by small, low projection at distomarginal edge of sternite 5 (adjacent to sternite 4) with shallow depression.
Pleon narrow, slender, long ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 3D View FIGURE 3 , 5F, G View FIGURE 5 ); somite 1 longitudinally narrow, wide ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 3D View FIGURE 3 , 4B View FIGURE 4 , 5F, G View FIGURE 5 ); somite 2 yoke-like, reaching coxae of fourth ambulatory leg ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 4B View FIGURE 4 , 5F, G View FIGURE 5 ); somites 3–6 fused, forming elongate trapezoidal plate, very shallow suture just visible between somites 3 and 4, surface smooth ( Figs. 3D View FIGURE 3 , 4B View FIGURE 4 , 5F, G View FIGURE 5 ); somite 6 longitudinally subrectangular, free, surface unarmed, lateral margins gently sinuous to gently convex ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 3D View FIGURE 3 , 4A View FIGURE 4 , 5F, G View FIGURE 5 ); telson triangular, longer than wide ( Figs. 1E View FIGURE 1 , 2E View FIGURE 2 , 3D View FIGURE 3 , 4A View FIGURE 4 , 5F, G View FIGURE 5 ).
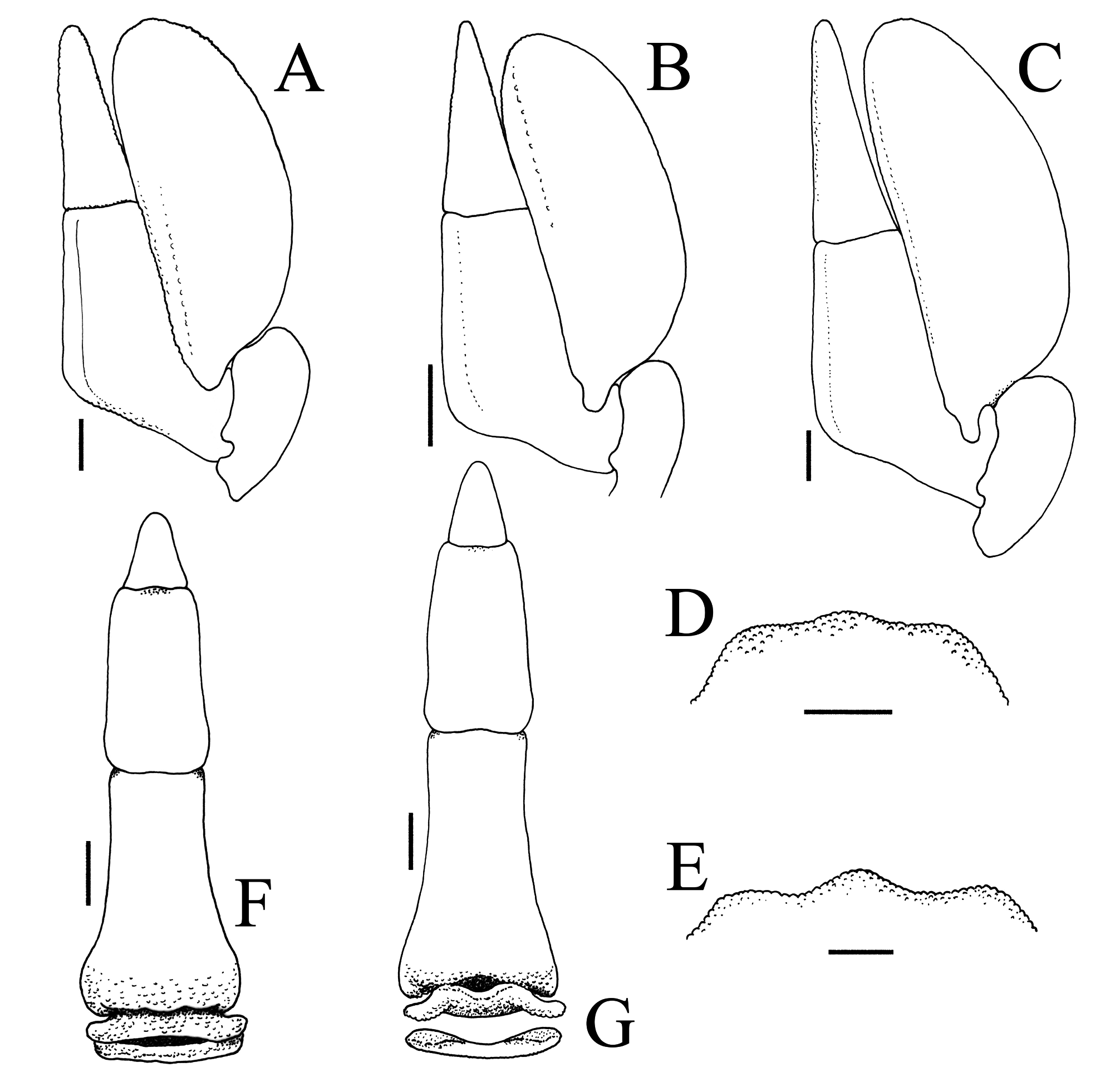
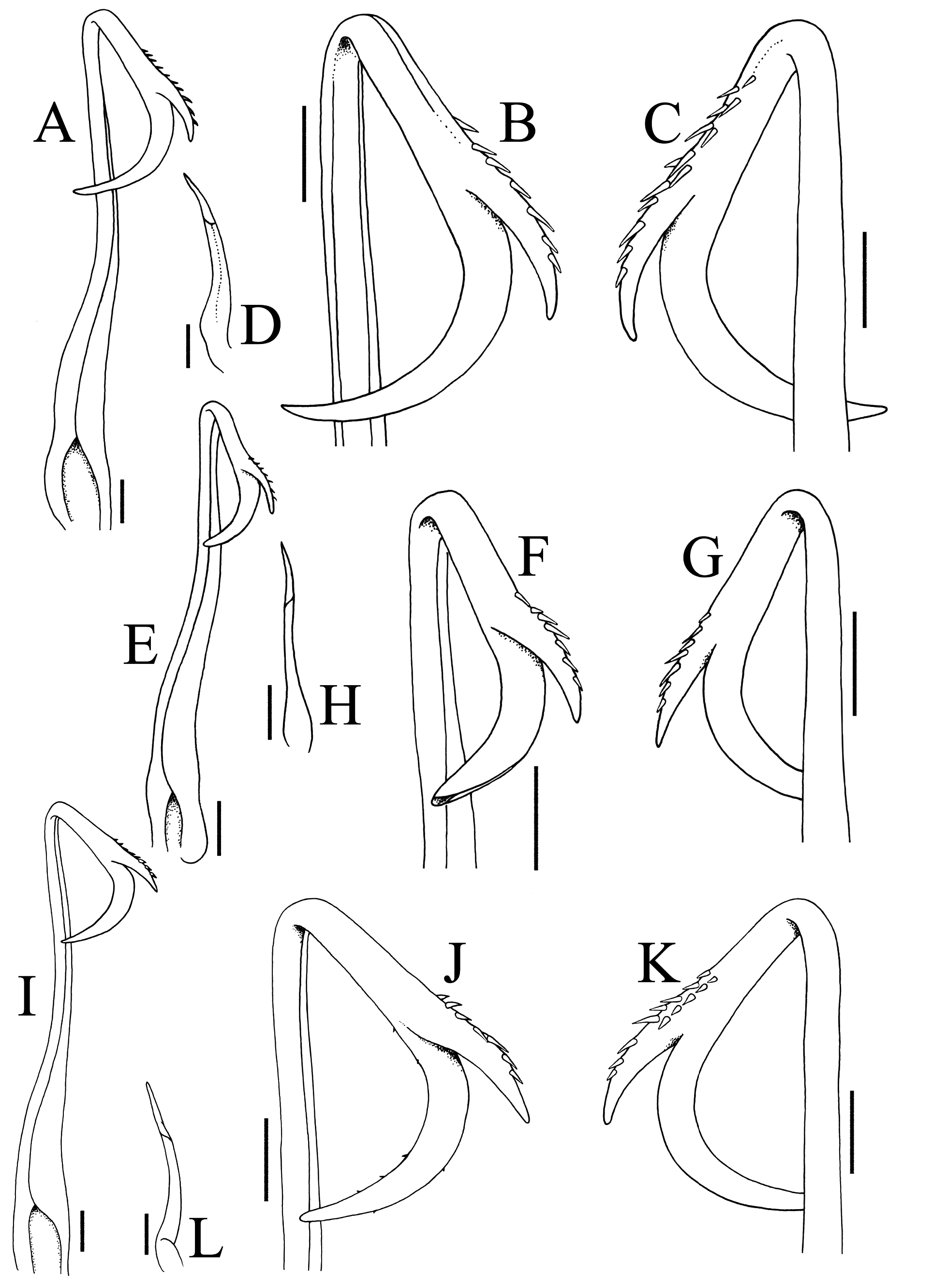
G1 elongate, slender, shaft gradually tapering distally; distal quarter sharply bent backwards 120–150º from the longitudinal axis; with curved, tapering subdistal process, outer margin lined with 1 or 2 rows of 5–8 spines; distal part elongate, curved, hook-shape, reaches to, overlaps or overreaches shaft, opening distal ( Fig. 6A–C, E–G, I–K View FIGURE 6 ). G2 short, ca. one-third length of straight part of G1; apex elongate, subspatuliform ( Fig. 6D, H, L View FIGURE 6 ).
Females. The carapaces of the female specimens are similar to the males in all aspects, but the chelipeds are proportionately shorter ( Fig. 7A, D View FIGURE 7 ). The female pleon is longitudinally ovate, with somites 1 and 2 free, somites 3–6 completely fused to form a domed plate that completely covers the thoracic sternum ( Fig. 7B, E View FIGURE 7 ). The telson is triangular and mobile ( Fig. 7B, E View FIGURE 7 ). The vulvae are large, obliquely ovate and positioned on the anterior part of sternite 6, without any sign of a sternal vulvar cover ( Fig. 7C, F View FIGURE 7 ).
Colour. Targioni-Tozzetti (1877: 203) described the fresh colours: “Non ho che un solo maschio perfetto, e una femmina in istato di muta assai guasta; il primo è colorato in giallastro e coperto come di una patina bruno fuliginosa, che lascia scoperte le zampe rosso-brune, e i tarsi giallo-crocei.” [I have only one perfect male, and one female in which is a poor state due to imperfect moulting; the first is yellowish colored and covered as with a sooty brown patina which covers the reddish-brown and reticulated yellow legs.] This “sooty appearance” as described by Targioni-Tozzetti (1877) is very obvious in several of the specimens from Malaysia and Singapore (see Figs. 3A, B View FIGURE 3 , 7D View FIGURE 7 ). In life, the specimens from Singapore are greyish-brown.
Remarks. Philyra fuliginosa was described by Targioni-Tozzetti (1877) from a male (6.5 × 8.0 mm) and a female (7.0 × 8.5 mm) from Java. The present specimens agree with the type description and figures well, the only apparent difference being in the proportions of the exopod of the third maxilliped. That figured by Targioni-Tozzetti (1877: fig. 3g) shows a relatively more slender exopod but this is wider in the present specimens ( Figs. 1C View FIGURE 1 , 5A View FIGURE 5 ). This may just be because they were depicted in situ; in life the exopod fits onto the sloping sides of the pterygostomial region and appear more slender than they actually are. Rathbun (1909: 108) described P. olivacea from one male (9.0 × 7.8 mm) collected by a seine from the coast of Lem Ngob in the Gulf of Thailand. She later described the species in more detail ( Rathbun, 1910), providing a photograph of the specimen and figures of the carapace, third maxillipeds and pleon. Rathbun (1910: 313) commented that O. olivacea was close to O. fuliginosa but the latter differed in “in wanting a postero-lateral angulation, and in the form of the ♂ abdomen and chela.” Comparing their published figures, the differences would be that in O. fuliginosa , the posterior carapace margin has two low lateral lobes ( Targioni-Tozzetti 1877: pl. 12 fig. 3a) (versus with 2 prominent lobiform teeth in O. olivacea ; Rathbun, 1910: p1. 11, fig. 17); the lateral margin of male pleonal somite 6 is sinuous ( Targioni-Tozzetti 1877: pl. 12 fig. 3c) (versus gently convex in O. olivacea ; Rathbun, 1910: fig. 4c); and the fingers of the chela are more strongly bent (Targioni-Tozzetti 1877: pl. 12 fig. 3g) (versus less bent in O. olivacea ; Rathbun 1910: p1. 11, fig. 17).
In the ZRC are two lots which have originally been identified as P. fuliginosa , both from Peninsular Malaysia, one from Muar in Johor (ZRC 1970.8.8.7) ( Yang, 1979) and another from Jeram in Selangor (ZRC 2020.0345). There are also several lots from Peninsular Malaysia and Singapore which were identified as P. olivacea by the late H.-L. Chen. A re-examination of this material showed that the specimens from Jeram (ZRC 2020.0345) agreed very well with the type description and figures of P. fuliginosa by Targioni-Tozzetti (1877), with the rest of the material (including the Muar specimen, ZRC 1970.8.8.7) matching that for P. olivacea as described and figured by Rathbun (1909, 1910), Dai et al. (1986), Dai & Yang (1991), Tan (1995) and Chen & Sun (2002) very well.
Several characters appear to differentiate the specimens from Jeram (ZRC 2020.0345) from the rest of the material, notably in the proportionately shorter cheliped merus and ambulatory merus ( Fig. 1A, F View FIGURE 1 ); the lateral margin of male pleonal somite 6 is slightly sinuous ( Figs. 1E View FIGURE 1 , 4A View FIGURE 4 , 5F View FIGURE 5 ); and the distal projection of the G1 prominently overreaches the main shaft of the ( Fig. 6A–C View FIGURE 6 ). These differences, however, are not significant at the species level.
The male cheliped merus of the Jeram P. fuliginosa is proportionately shorter ( Fig. 1A View FIGURE 1 ) as figured by Targioni-Tozzetti 1877: pl. 12 fig. 3g), and this structure is distinctly longer in most of the specimens of P. olivacea examined ( Figs. 2A View FIGURE 2 , 3A, B View FIGURE 3 ), even when specimens of similar sizes are compared. In a few smaller specimens of P. olivacea , however, the merus is somewhat shorter ( Fig. 3C View FIGURE 3 ). The same pattern is observed for female specimens ( Fig. 7A View FIGURE 7 versus Fig. 7D View FIGURE 7 ). Slight differences in proportions of the cheliped merus, however, can be explained by variation. The ambulatory merus of the Jeram male is proportionately shorter ( Fig. 1F View FIGURE 1 ) and looks identical to that figured by Targioni-Tozzetti (1877: pl. 12 fig. 3b). This is also true for the female specimen from Jeram ( Fig. 7A View FIGURE 7 versus Fig. 7D View FIGURE 7 ). Most of the specimens of P. olivacea have the ambulatory merus slightly longer ( Fig. 2F View FIGURE 2 ), but we observe that larger specimens tend to have more slender legs, although not always the case. The differences, observed, in any case, are minor and are unlikely to be reliable at the species level.
The differences in the male pleonal somite 6 and G1 appear more significant. Most of the specimens of P. olivacea have the lateral margins of male pleonal somite 6 gently convex ( Figs. 2E View FIGURE 2 , 3D View FIGURE 3 , 5G View FIGURE 5 ) and the distal projection of the G1 reaches or overlaps the main shaft but does not overreach it ( Fig. 6E–G, I–K View FIGURE 6 ). A male (6.8 × 8.0 mm, ZRC 2014.0176) from Singapore is a typical P. olivacea with regards to the frontal lobe, proportions of the cheliped merus, ambulatory merus and third maxilliped exopod, with the lateral margins of pleonal somite 6 clearly convex, but its G1 distal process overlaps the main shaft by a short distance, albeit not to the degree of the Jeram P. fuliginosa ( Fig. 6A–C View FIGURE 6 ). Another male (11.2 × 14.0 mm, ZRC 2009.0217) of P. olivacea has the proportionately longer cheliped merus, ambulatory merus and narrower third maxilliped exopod like the other specimens in the lot except that the lateral margins of its pleonal somite 6 are sinuous and the G1 has the distal process slightly overlapping the main shaft.
In addition, the frontal median lobe is relatively lower in P. fuliginosa ( Fig. 5D View FIGURE 5 ) while in most of the specimens of P. olivacea , the frontal median lobe is usually more developed ( Fig. 5D View FIGURE 5 ), but this appears to be partly associated with size as smaller specimens tend to have somewhat lower frontal lobes. The exopod of the third maxilliped is relatively wider in the specimens of P. fuliginosa ( Fig. 5A View FIGURE 5 ) and while most of the specimens of P. olivacea have a slightly narrower exopod ( Figs. 3E View FIGURE 3 , 5B View FIGURE 5 ), this is not always the case with a few also possessing a wider one ( Fig. 5C View FIGURE 5 ).
As for the three characters used by Rathbun (1909, 1910) to separate P. olivacea from P. fuliginosa , it was discussed earlier that the form of the male pleon varies.As to the strength of the tooth at the angles of the posterior carapace margin, this character varies substantially, from low ( Figs. 1B View FIGURE 1 , 3C View FIGURE 3 ) to medium size ( Fig. 2B View FIGURE 2 , 3A View FIGURE 3 ) to strong ( Fig. 3B View FIGURE 3 ). The same applies to the slightly different degree of bending of the cheliped fingers varies (e.g., Fig. 3A–C View FIGURE 3 ).
In summary, there are no clear differences between P. fuliginosa Targioni-Tozzetti, 1877 , and P. olivacea Rathbun, 1909 , and the author therefore synonymises the two taxa. The types of P. fuliginosa are not extant and almost certainly lost. Lucas (1981: 200) searched for them for his study on hymenosomatids and commented they were lost in the Second World War, and a fresh search has confirmed this (see also Schubart & Ng 2020: 956). There is no immediate need to select a neotype as the specimens on hand from Malaysia and Singapore are clearly P. fuliginosa .
Ecology. The Singapore specimens were all collected from the southern part of the island, in sandy-muddy habitats in shallow water less than 20 m deep. Two specimens of O. fuliginosa were obtained from an unusual habitat, a polychaete reef in Jeram, Malaysia (ZRC 2020.0345), where a number of new decapods were also collected (see Polgar et al. 2015), including two rare species of porcellanid crabs (Osawa et al. 2018; Osawa & Ng 2018).
Distribution. The species has been reported from Java, Peninsular Malaysia, Singapore, Gulf of Thailand, to Zhejiang, Fujian and Hainan Island in southern China ( Targioni-Tozzetti 1877; Rathbun 1909, 1910, 1931; Yang 1979; Dai et al. 1986; Dai & Yang 1991; Tan 1995; Chen & Sun 2002).
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
SuperFamily |
Leucosioidea |
|
Family |
|
|
SubFamily |
Ebaliinae |
|
Genus |
Ovilyra fuliginosa ( Targioni-Tozzetti, 1877 )
| Ng, Peter K. L. 2021 |
Philyra aff. fuliginosa
| Yang, C. M. 1979: 5 |
Pseudophilyra olivacea
| Shen, C. J. & Dai A. Y. 1964: 29 |
Philyra olivacea
| Galil, B. S. 2009: 281 |
| Ng, P. K. L. & Guinot, D. & Davie, P. J. F. 2008: 93 |
| Chen, H. & Sun, H. 2002: 401 |
| Dai, A. - Y. & Yang, S. - L. 1991: 91 |
| Dai, A. - Y. & Yang, S. - L. & Song, Y. - Z. & Chen, G. - X. 1986: 81 |
| Serene, R. 1968: 46 |
| Rathbun, M. J. 1931: 99 |
| Rathbun, M. J. 1910: 312 |
| Rathbun, M. J. 1909: 108 |
Philyra fuliginosa
| Galil, B. S. 2009: 281 |
| Ng, P. K. L. & Guinot, D. & Davie, P. J. F. 2008: 93 |
| Serene, R. 1968: 46 |
| Targioni-Tozzetti, A. 1877: 201 |