Catapaguropsis brucei, Mclaughlin, Patsy A. & Lemaitre, Rafael, 2007
|
publication ID |
https://doi.org/10.5281/zenodo.178328 |
|
DOI |
https://doi.org/10.5281/zenodo.6236537 |
|
persistent identifier |
https://treatment.plazi.org/id/03EE8782-5266-565B-FF1C-22A48C9B7FB3 |
|
treatment provided by |
Plazi |
|
scientific name |
Catapaguropsis brucei |
| status |
sp. nov. |
Catapaguropsis brucei View in CoL n. sp.
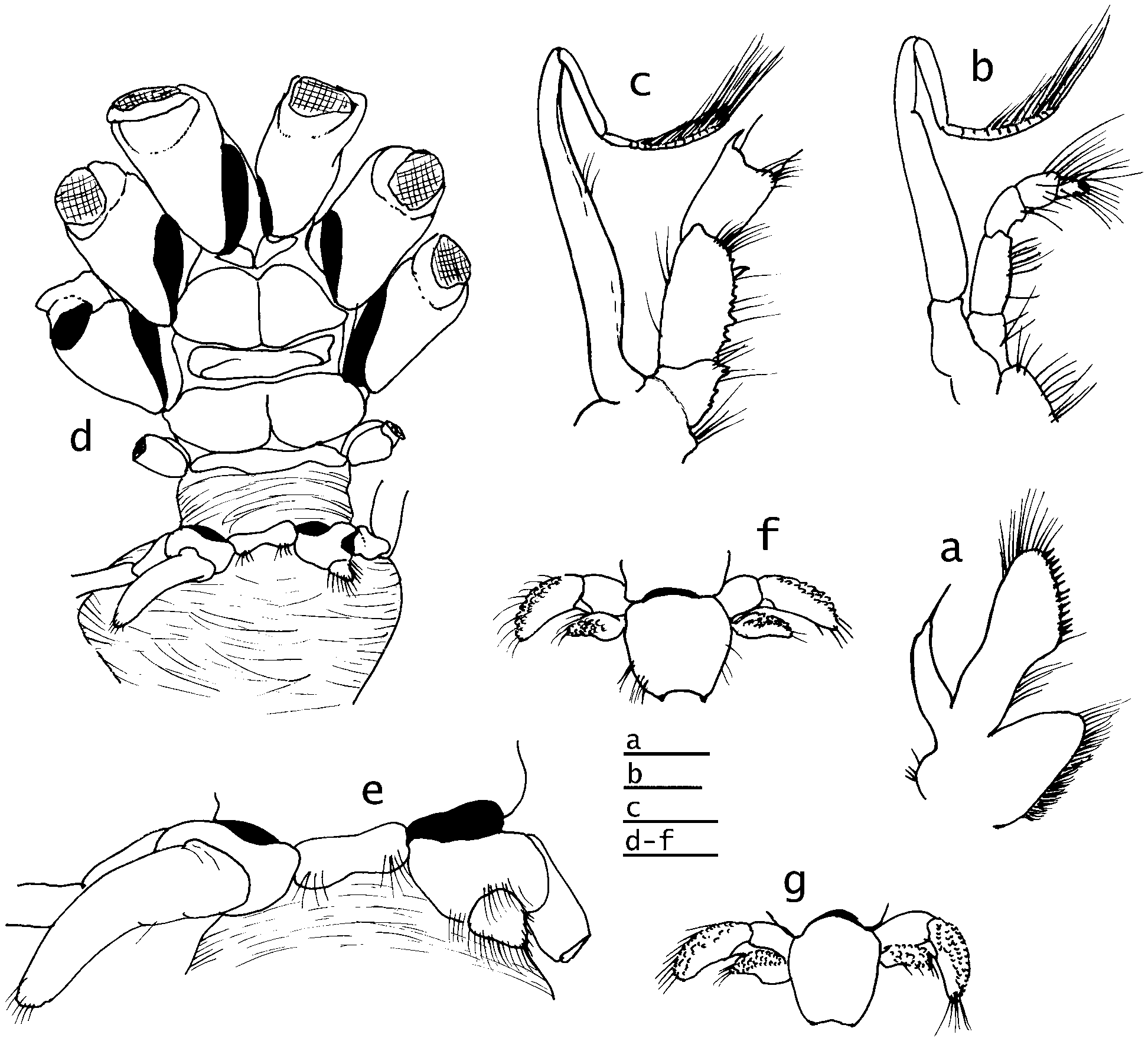
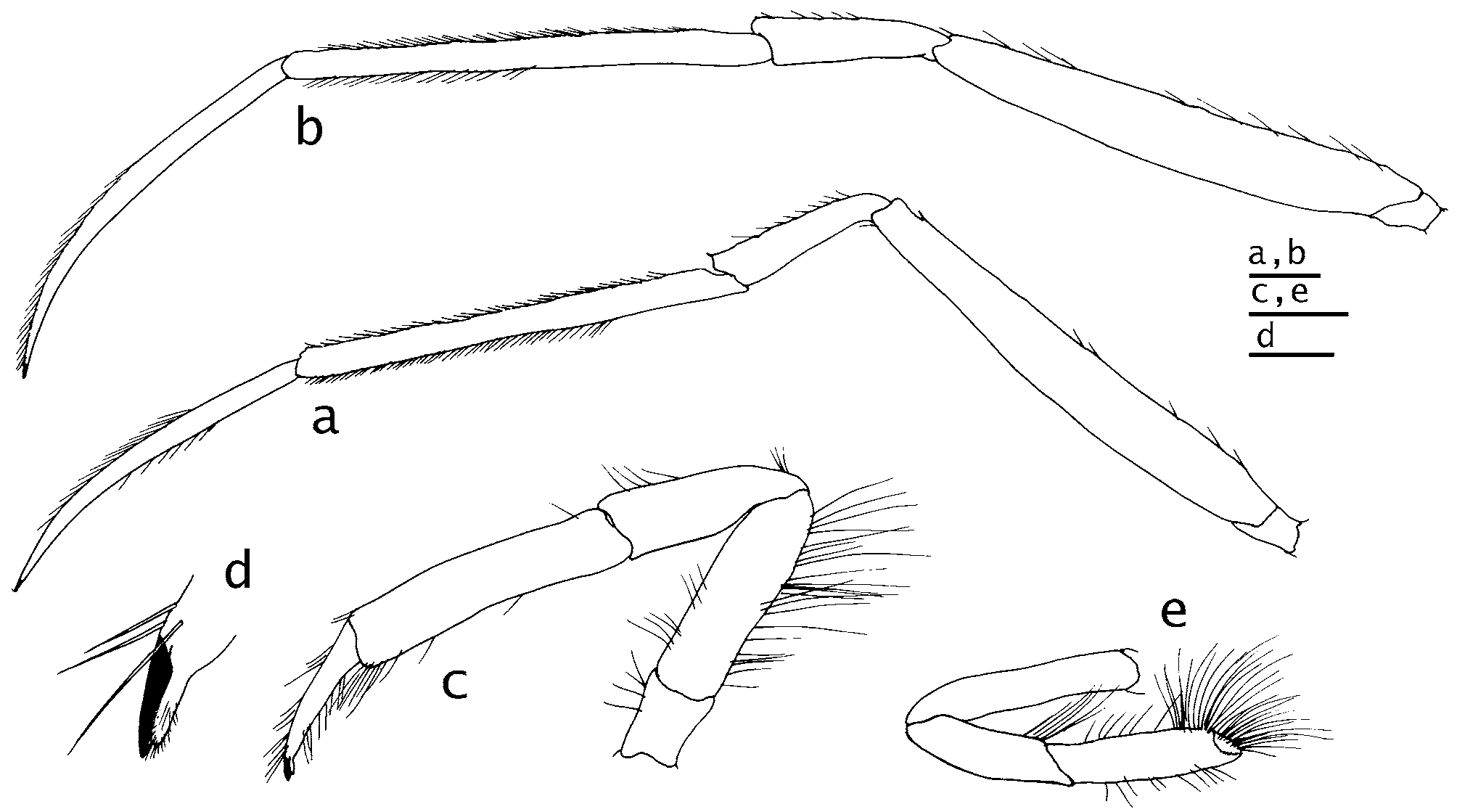
( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 d,e, 3, 4)
Type material. South China Sea. Holotype female (sl = 2.4 mm), Cruise 4/65 stn 84, trawl 347, 12º02’N, 112º49’E, 366–388 m, 12 Mar 1965, coll. A.J. Bruce ( MNHN Pg 7735). Paratype. Male (sl = 2.7 mm, missing second through fourth left and third right pereopods), same data as holotype ( MNHN Pg 7736).
Description. Shield ( Fig. 1 View FIGURE 1 a) somewhat vaulted, broader than long, moderately well calcified; anterior margin between rostral lobe and lateral projections concave; anterolateral margins sloping; posterior margin roundly truncate. Rostral lobe broadly rounded or roundly subtriangular, not produced beyond level of broadly rounded, unarmed lateral projections. Dorsal surface of shield with 2 subrostral, semiovate swellings and few sparse setae (not shown in Fig. 1 View FIGURE 1 a). Carapace lateral lobes narrow, not reaching 0.5 of shield in holotype, but reaching beyond in male paratype. Posterior carapace short, with broad median plate; cardiac sulci reaching to posterior margin. Branchiostegites membranous, unarmed.
Ocular peduncles very short, 0.6–0.7 length of shield, very slender penultimate segments visible dorsally, ultimate segments broadly expanded at bases of corneas; corneal diameter approximately equal to total peduncular length (including cornea). Ocular acicles very small, triangular, each terminally acute; separated basally by more than three times basal length of one acicle.
Antennular peduncles overreaching distal margins of corneas by approximately 0.5 length of penultimate peduncular segments; ultimate segment with several long setae at dorsodistal margin laterally; penultimate segment glabrous; basal segment with unarmed dorsolateral margin.
Antennal peduncles overreaching distal corneal margins by approximately 0.5 length of ultimate segments. Fifth and fourth segments unarmed; third segment with sparse tuft of setae on ventrodistal margin; second segment with dorsolateral distal angle produced, terminating in small spine, dorsomesial angle with small spine; first segment unarmed. Antennal acicle reaching beyond distal margin of fourth peduncular segment, slender, terminating in simple spine. Antennal flagella missing. Third maxilliped with tiny spinule on dorsodistal margin of merus.
Chelipeds subequal in length in female, left exceeding right in distal extension in male by about length of dactyl; right appreciably stouter in both sexes; each lacking hiatus between dactyl and fixed finger. Right cheliped ( Fig. 3 View FIGURE 3 a–c) with chela 2.6 (male) to 3.5 (female) as long as broad. Dactyl 0.3 (male) to 0.4 (female) length of palm; dorsomesial margin rounded, dorsal surface weakly convex, all surfaces unarmed but with numerous scattered, moderately long setae ventrally; cutting edge serrate, with 2 low, broad, calcareous teeth, terminating in tiny corneous claw, slightly overlapped by fixed finger. Palm approximately equal to carpus in female, 0.4 longer than carpus in male, dorsomesial and dorsolateral margins rounded and unarmed, dorsal surface weakly convex, also unarmed, fixed finger similarly unarmed but ventral surfaces of both palm and fixed finger with numerous moderately long setae; cutting edge of fixed finger serrate, with 2 broad, low calcareous teeth and few much smaller distal calcareous teeth, terminating in tiny corneous claw. Carpus slightly shorter to slightly longer than merus; dorsomesial and dorsolateral margins each with row of irregularly-sized spines, largest on dorsomesial margins in both sexes, but spines of female largest; female also with 1 much larger spine at each dorsodistal angle; ventromesial and ventrolateral margins unarmed in female, male with few minute granules on ventrolateral margin. Merus laterally compressed; male with prominent spine on dorsodistal margin, row of low protuberances and few setae on dorsomesial margin, ventromesial and ventrolateral margins each with row of tiny spines; female with small spine on dorsodistal margin, other margins and surfaces unarmed, but with few setae dorsally. Ischium unarmed in both sexes.
Left cheliped ( Fig. 3 View FIGURE 3 d–f) long and very slender; left chela 5.7 (male) to 6.4 (female) as long as broad. Dactyl approximately 1.5 longer than palm; surfaces rounded and unarmed but with sparse, moderately short setae distally; cutting edge with row of tiny, calcareous teeth, terminating in minute corneous claw. Fixed finger similarly rounded and unarmed but with sparsely scattered setae; cutting edge with tiny calcareous teeth interspersed with minute corneous teeth, terminating in very small corneous claw and very sparse tuft of short setae. Palm with convex dorsal surface unarmed and glabrous; dorsomesial and dorsolateral margins rounded. Carpus and merus both noticeably longer than palm, but only slightly longer than dactyl. Carpus of female holotype with row of moderately widely-spaced small spines, 1 much larger spine on each dorsodistal angle, dorsolateral margin with row of smaller spines; male with even smaller spines on dorsomesial and dorsolateral margins, smallest laterally. Merus with very small dorsodistal spine in both sexes; surfaces unarmed; ventromesial and ventrolateral margins not delimited. Ischium unarmed.
Second and third pereopods ( Fig. 4 View FIGURE 4 a, b) long, slender, terminating in slender corneous claws. Dactyls 0.7– 0.8 length of propodi, not blade-shaped in either sex; dorsal and ventral surfaces each with row of moderately long setae. Propodi 2.2–2.3 length of carpi; dorsal surfaces each with dorsal row of very small spinulose protuberances, each with short, stiff bristle, ventral surfaces each with row of fine setae. Dorsal margins of carpi each with row of spinulose protuberances accompanied by short bristles, dorsodistal margins each with very small spine, smallest on third pereopods. Meri 2.4–2.5 length of carpi, each dorsally with minute protuberances accompanied by spiniform setae. Ischia unarmed. Fourth pereopods ( Fig. 4 View FIGURE 4 c, d) simple, without propodal rasps; preungual processes nearly as long as dactylar claws; meri each with long setae dorsally and ventrally, densest dorsally. Fifth pereopods ( Fig. 4 View FIGURE 4 e) minutely chelate. Sternites of second and third pereopods each with median concavity; anterior lobe of third subrectangular in both sexes.
Male with coxae of fifth pereopods approximately equal, right with stout, short sexual tube appearing as posterior coxal extension ( Fig. 2 View FIGURE 2 d, e) directed toward exterior; coxa of left with very short tube directed posteriorly; pleon bulbous anteriorly, markedly reduced posterior to position of pleopod 4; no paired or unpaired pleopods. Female with paired gonopores; without paired, modified first pleopods, pleon similarly bulbous anteriorly, reduced posterior to pleopod 4, left pleopods 2–4 well developed, biramous, no unpaired fifth pereopod; non-eyed eggs 0.5–0.6 mm diameter.
Uropods and telson ( Fig. 2 View FIGURE 2 f, g) symmetrical in both sexes, markedly reduced. Telson with slight lateral incisions or indentations separating anterior and posterior portions; posterior lobes separated by minute median cleft; terminal margins horizontal, with 1 or 2 microscopic spinules.
Etymology. It is a pleasure to dedicate this species to its collector, the noted carcinologist, Dr. Alexander J. (Sandy) Bruce.
Color. Unknown.
Habitat. The carcinoecium ( Fig. 1 View FIGURE 1 c, d) found with the male is a Crepidula -like chitinous pseudoshell with a small piece of calcified shell remaining posteriorly. Although no tissue was found with this carcinoecium, the chitinous portion is likely to have been produced by a cnidarian, probably an actinian. Chitinous pseudoshells are produced by a number of actinians and zoanthids that associate with various deep-water hermit crab species (e.g., Fautin Dunn et al. 1980; Lemaitre 1989, 2004). The carcinoecium was disassociated with the male when the specimen was examined, but its size suggests that it covered only the uropods and telson of the animal.
The female’s carcinoecium, while still in place on the specimen ( Fig. 1 View FIGURE 1 a), is slightly larger and consists of a cnidarian of unknown identity, probably an actinian (A.G. Collins, personal communication), covering a gastropod shell of the family Architectonicidae . Several spermatophores, presumably from a male of this new species, were found attached near the anterior margin of the carcinoecium, and several more were also attached on the interior wall of the carcinoecium (hidden from dorsal view, Fig. 1 View FIGURE 1 a) (C.C.Tudge, personal communication). No evidence of a pseudoshell was found with this carcinoecium, which covers only the uropods and telson.
Distribution. Known only from the type locality.
Variation. The marked sexual dimorphism apparent in C. queenslandica appears to be absent in C. brucei , although additional specimens may show that the male second and third pereopods are dissimilar in length as they are in the former species.
Remarks. Had Catapaguropsis not initially been proposed for the sexually dimorphic and similarly female characters of C. queenslandica , the relationship of Catapaguropsis to Catapagurus might not have been immediately recognizable. The only significant characters that C. brucei n. sp. shares with some species of Catapagurus are the short, stout ocular peduncles, elongate chelipeds and ambulatory legs, finely spinose carpi of the chelipeds, broad thoracic sternites 2 and 3, and the male right sexual tube, but none of these characters are mutually exclusive for the two genera. As indicated by Lemaitre & McLaughlin (2006), the shared male characters suggest a much closer relationship with Pteropagurus , despite differences in pleon reduction; however, neither are these characters mutually exclusive to those two genera.
As previously noted, the carcinoecia of C. queenslandica are not known. Lemaitre & McLaughlin (2006) speculated that they could differ for males and females because of the marked reduction of the posterior pleon, uropods and telson in the single known male of that species. That the reduction did not result from injury or abnormal development has been clearly shown by the similar reductions seen in both sexes of C. brucei n. sp. Nonetheless, it is uncertain whether the different carcinoecia occupied by the male and female of the latter species reflect actual selection differences or simply availability. Both carcinoecia protect only the posterior portions of the pleons, uropods and telsons ( Fig. 1 View FIGURE 1 c, d). The relatively large clutch of developing eggs of the female of C. brucei n. sp. is completely exposed.
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |