Glaphyrosoma stephanosoltis Richardson, Trimm, Paderes, Koehl
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4671.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:BEC614E3-13ED-482A-845E-211C242B7D10 |
|
persistent identifier |
https://treatment.plazi.org/id/03EE878B-3970-B020-FF7C-FDFDFD96FF17 |
|
treatment provided by |
Plazi |
|
scientific name |
Glaphyrosoma stephanosoltis Richardson, Trimm, Paderes, Koehl |
| status |
|
Glaphyrosoma stephanosoltis Richardson, Trimm, Paderes, Koehl , & Song, sp. nov. Figs 1–11 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11
urn:lsid: Orthoptera .speciesfile.org:TaxonName:506875
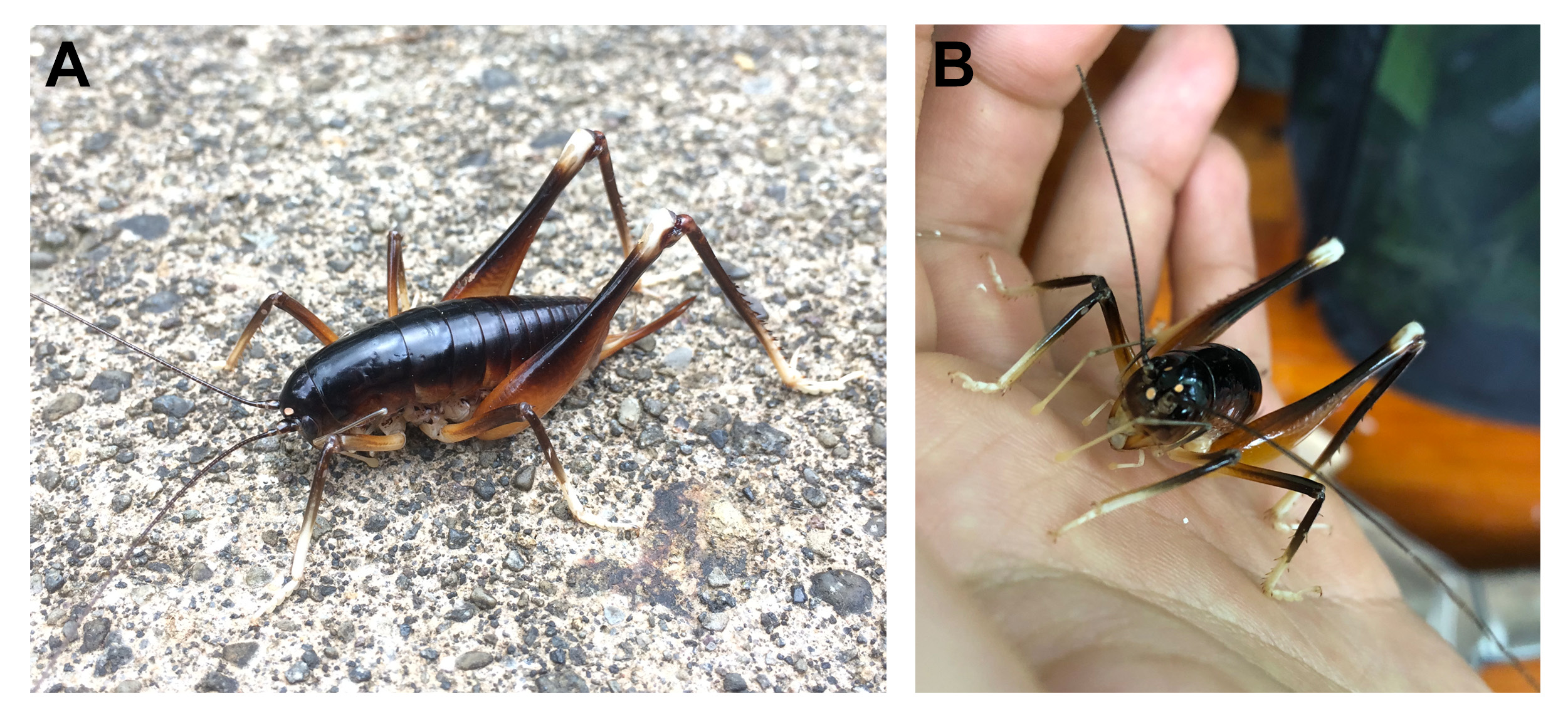
Diagnosis. Glaphyrosoma stephanosoltis sp. nov. is dorsally colored in solid dark brown ( Figs. 1 View FIGURE 1 , 3 View FIGURE 3 , 10 View FIGURE 10 ), and is similar in overall coloration to G. gracile , G. anderi , G. beretka , G. bulbosum , G. pushenkovi , G. franciscoasturiasi , G. hectorcentenoi , and G. magnaproctalis . However, it can be separated from all other members of the genus by having quadrangular male paraproctal process with a spine protruding medially facing upwards ( Figs. 7B, 7C View FIGURE 7 , 9A, 9D View FIGURE 9 ). This species is morphologically most similar to G. magnaproctalis according to the key presented by Cadena- Castañeda & Monzón-Sierra (2017), but differs in the position of the spine on male paraproctal process and the appearance of the male subgenital plate as well as the length of styli. The spine arises medially in G. stephanosoltis sp. nov. instead of being located at the top of the paraproctal process. The subgenital plate also shows more curvature between the styli forming a deep “V” shaped notch half the length of the subgenital plate ( Fig. 7D View FIGURE 7 , 9B View FIGURE 9 ) and differs from the subgenital plate of G. magnaproctalis in which there is almost no notch in between the styli.
Coloration. Dorsal portion of head dark brown, nearing black; gena and frons dark brown; clypeus light yellow. Median ocellus and two upper ocelli pinkish cream white (when alive) with light beige rings ( Fig. 1 View FIGURE 1 ). Antennae dark brown. Labrum white (when alive); mandibles light brown at base and dark brown at the tip; maxillary palpi and labial palpi creamy white. Entire dorsal region from thorax to abdomen dark brown with no delineated coloration patterns. Lateral margin of thoracic and abdominal tergites lighter brown. Sternum yellow/brown. Fore femora and mid femora yellowish brown distally, and darker brown apically. Hind femora lighter brown distally, gradually becoming darker apically and dorsally, with apical end (knee) abruptly becoming creamy white ( Fig. 1 View FIGURE 1 ). All tibiae distally dark brown, apically creamy white. All tarsi creamy white.
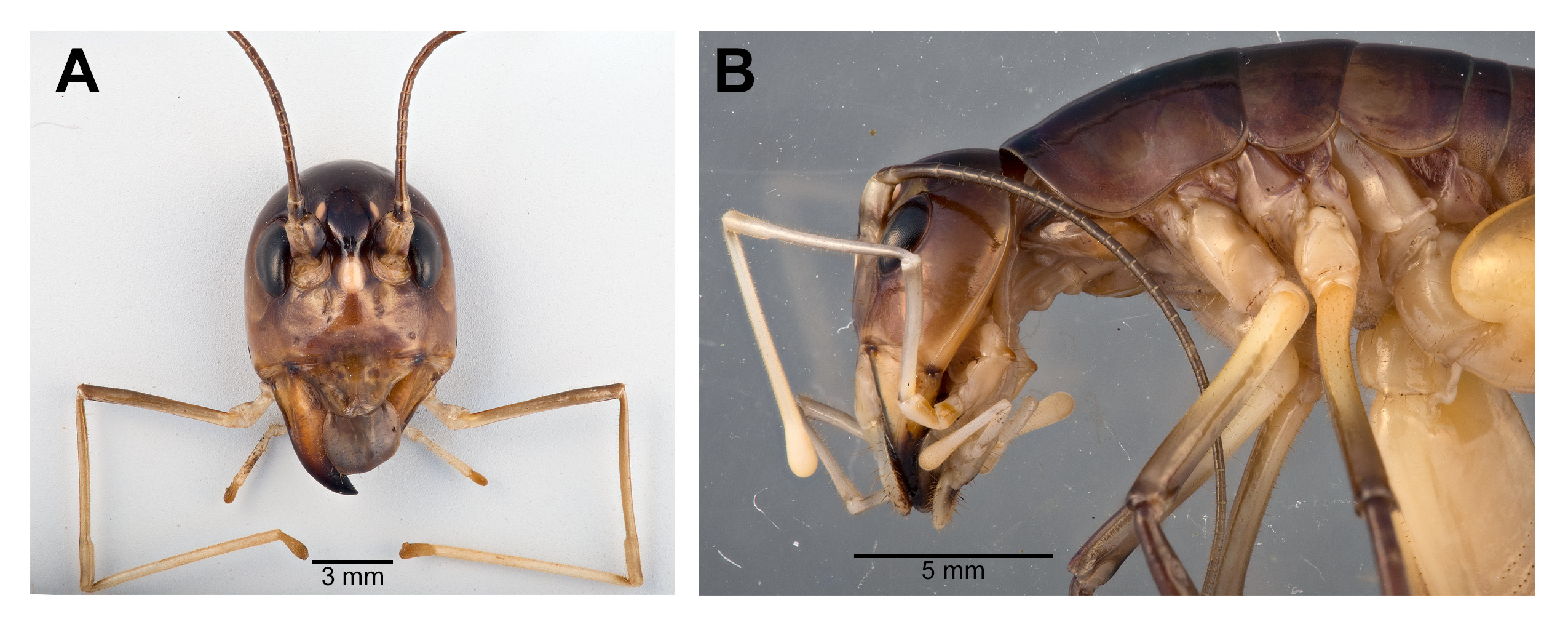
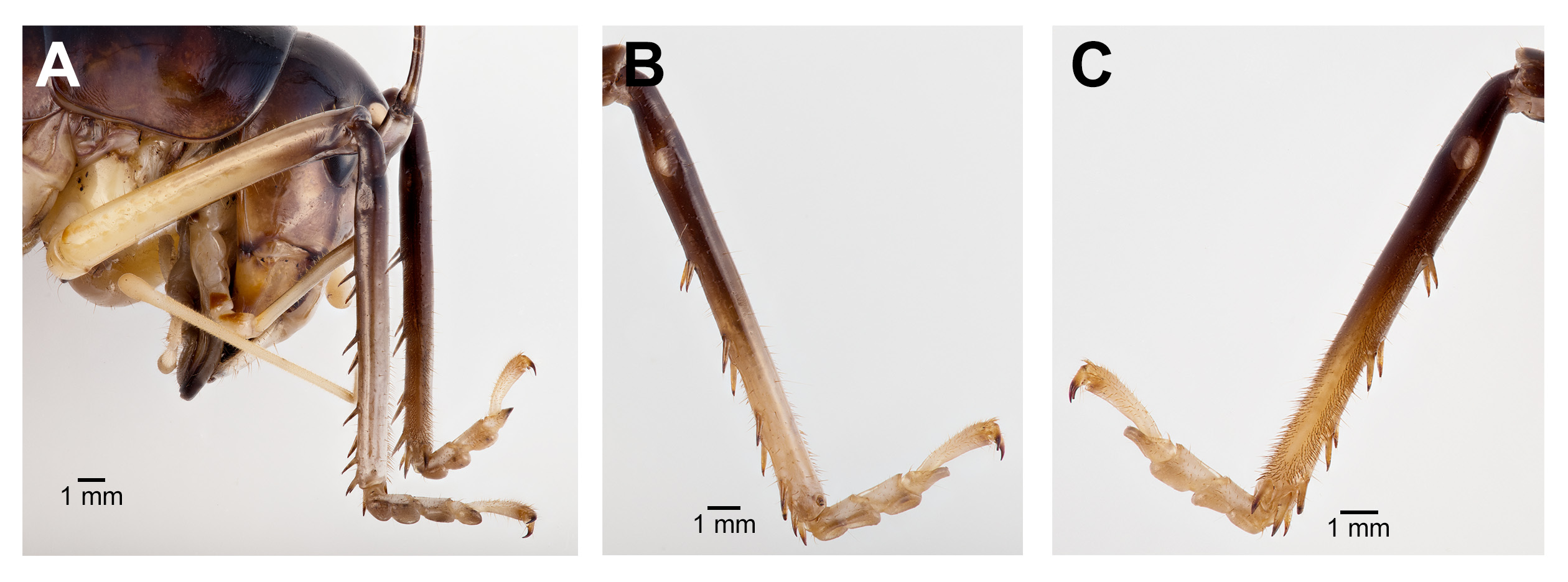
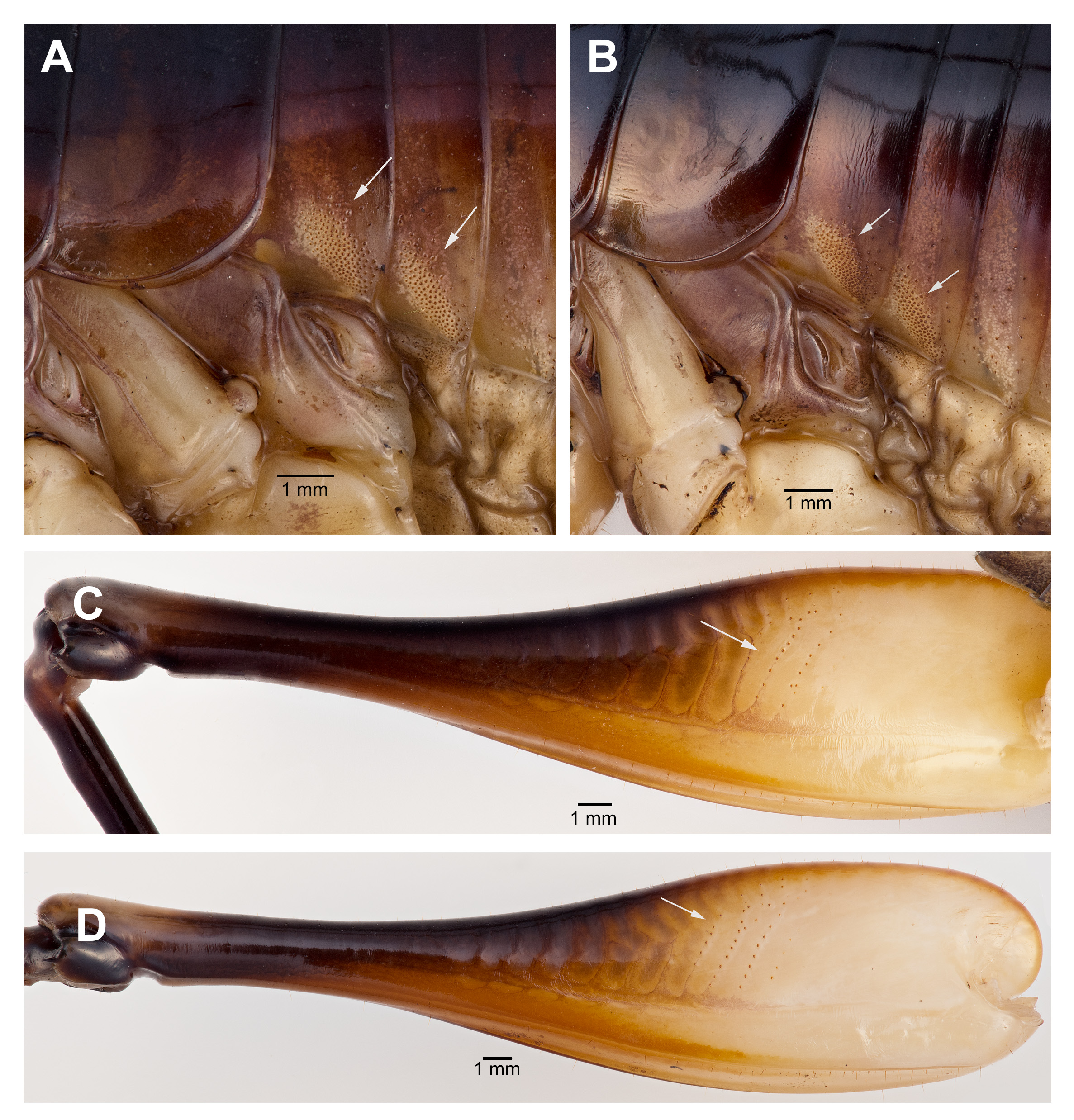
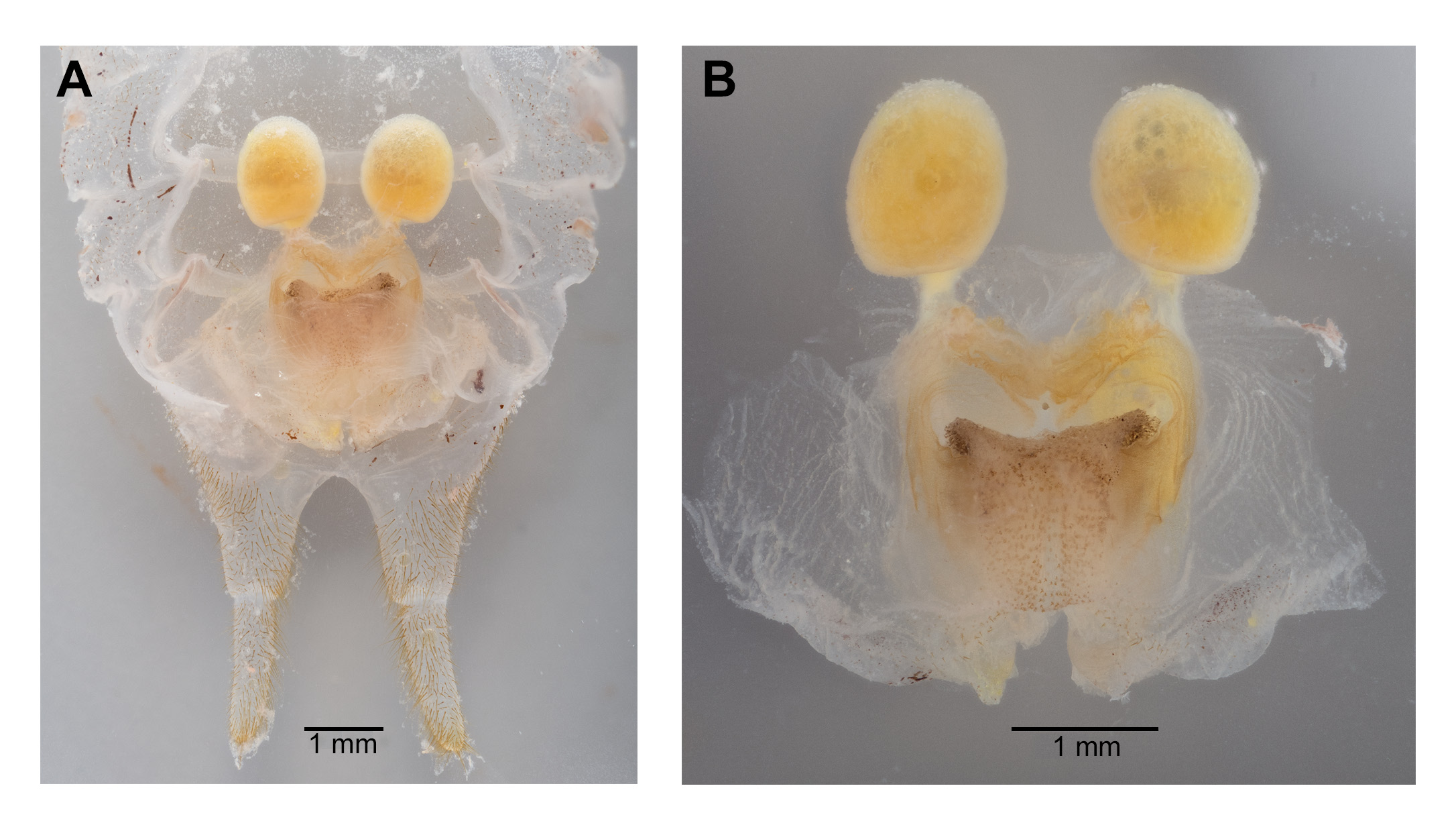
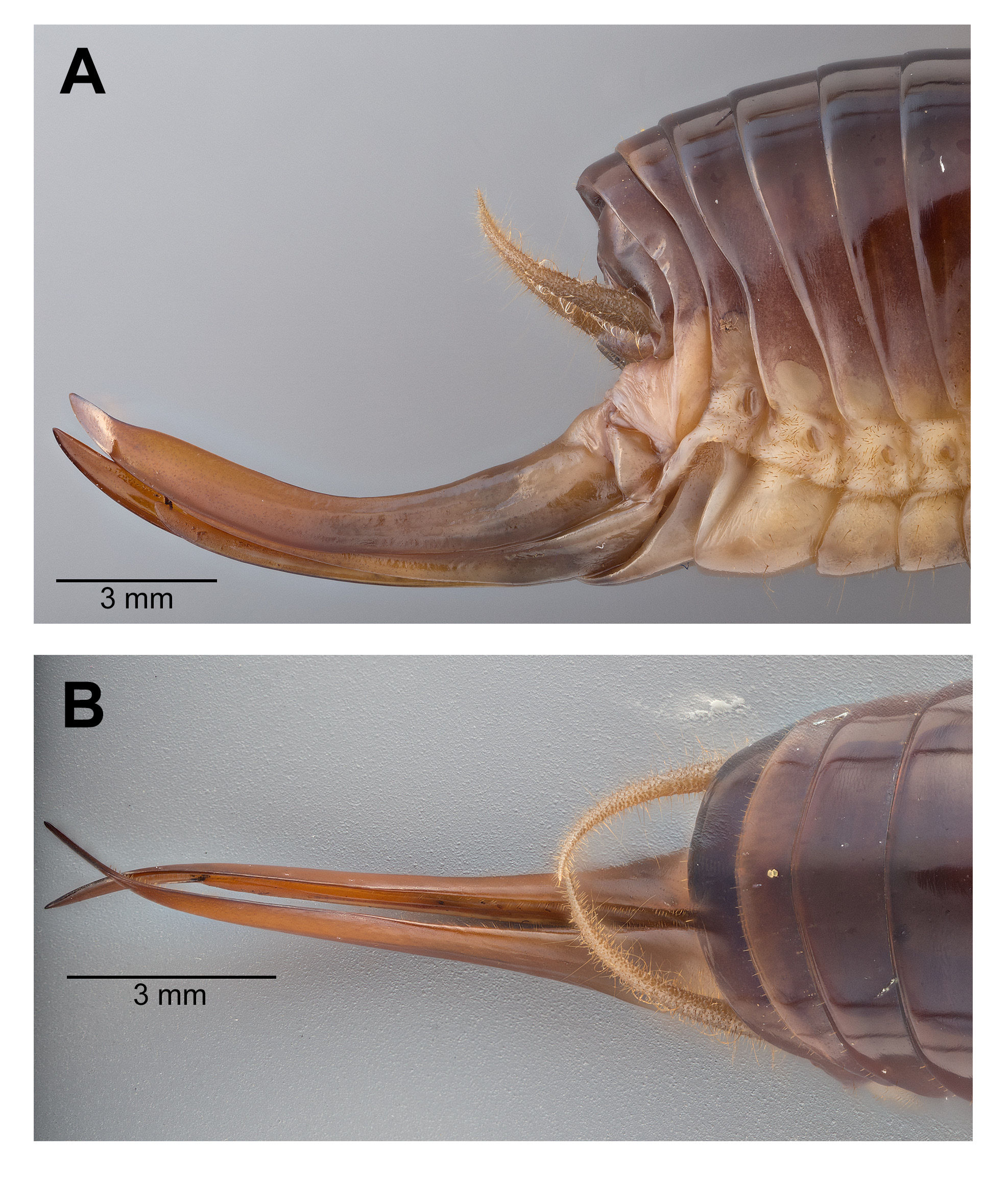
Male. Head ( Fig. 2A,B View FIGURE 2 ): Integument smooth. Vertex smooth without any depression, blended to frontal ridge. Gena convex. Frons, smooth, unpronounced, blended with the rest of head; two parallel depressions starting at the base of scape; a pair of small circular depressions above the mandibular condyles. Antennal scape originating from where frontal ocellus is positioned. Antenna filiform and at least three times longer than body. Ocelli round and not protruding from head. Eyes pear-shaped, narrowing dorsoventrally, half as long as the width of gena. Clypeus upside down triangular with broadly round ventrally, concave. Labrum circular. Mandibles narrowing to sharp end, articulation points pronounced below gena. Maxillary palpi very long, nearly three times the dorsoventral length of the head. Labial palpi evident, but not long, shorter than the length of mandible. Both maxillary and labial palpi with bulbous apical tips. Thorax: Integument smooth. Completely apterous. Pronotum about as long as wide ( Fig. 3A View FIGURE 3 ); sloping downward anteriorly; lateral lobe of pronotum square basally ( Fig. 2B View FIGURE 2 ). Mesonotal and metanotal lobes rounded distally ( Fig. 2B View FIGURE 2 ). Legs: Fore tibiae ventrally with 3 pairs of symmetrically arranged spines, 6 apical spurs composed of 1 pair of short symmetrical spines and 2 pairs of apical spurs ( Figs. 4A View FIGURE 4 , 5A View FIGURE 5 ); no subapical spines along the dorsal surface; tympanum present as depression at distal portion of inner and outer surface of fore tibiae ( Fig. 5A View FIGURE 5 ). Middle tibiae with 4 asymmetrical spines dorsally and 3 pairs of symmetrically arranged spines ventrally ( Fig. 4B View FIGURE 4 ); 6 apical spurs composed of 1 pair of short symmetrical spines, 1 pair of spurs dorsally, and 1 pair of spurs ventrally ( Fig. 4B View FIGURE 4 ). Hind tibiae dorsally armed with 18–21 unarticulated spines, 8 apical spurs composed of 1 pair of short spines, 1 pair of short spurs ventrally, two pairs of long spurs ( Fig. 4C View FIGURE 4 ). Hind femora absent of stripes or patterns ( Fig. 3A View FIGURE 3 ); light muscular lining visible through cuticle ( Fig. 3A View FIGURE 3 ); femoral groove present on outer side of hind femur from base to distal part ( Fig. 3A View FIGURE 3 ). Inner side of hind femora with 3–4 diagonal rows of stridulatory pegs present ( Fig. 6C View FIGURE 6 ). Abdomen: Integument smooth. Abdominal tergites gradually narrowing to last tergite ( Fig. 3B View FIGURE 3 ). Sternites rectangular and wider than its length. Lateral margin of first and second abdominal tergites with patches of granular stridulatory pegs ( Fig. 6A View FIGURE 6 ). The ninth tergite broadly bilobed and divided in the middle with a broad notch along the postero-medial margin ( Figs. 7C View FIGURE 7 , 9A, 9B View FIGURE 9 ). The tenth tergite forming a pair of hooks in the middle, which turn upward above the middle of the ninth tergite ( Figs. 7B, 7C View FIGURE 7 , 9A, 9C View FIGURE 9 ). Paraproct projected posteriorly but distal part still remains very close to last abdominal tergite and almost flat in appearance ( Figs. 7B View FIGURE 7 , 9A View FIGURE 9 ). Paraproctal process rectangular with a small denticle arising from medio-posteriorly lying almost flat and directed upwards ( Figs. 7B View FIGURE 7 , 9A, 9D View FIGURE 9 ). Subgenital plate with a prominent “V” shaped posteromedian notch and relatively long styli half the length of the subgenital plate ( Figs. 7D View FIGURE 7 , 9B View FIGURE 9 ). Cerci almost twice as long as the width of the tenth tergite, tapering from the base to the tip, arched inwards in dorsal view, covered in small hairs ( Figs. 7A, C, D View FIGURE 7 ). Male internal genitalia largely membranous and as in Fig. 8 View FIGURE 8 .
Female. Similar to male ( Fig. 10 View FIGURE 10 ). Subgenital plate triangular with a broad base and tapering toward the tip ( Fig. 9E View FIGURE 9 ). Ovipositor curved medially and apically directed ( Fig. 11 View FIGURE 11 ) and almost one-third length of hind femora. Paraproctal process absent. Cerci thicker than male cerci at base with prominently tapering to the tip ( Fig. 11 View FIGURE 11 ).
Measurements (in mm). Male (n=5): pronotum length 8.94–9.42 (9.24 ± 0.22); fore femur length 11.89–12.62 (12.23 ± 0.29); mid femur length 12.09–13.63 (12.87 ± 0.59); hind femur length 29.36–31.31 (30.02 ± 0.89); hind femur width 6.71–6.83 (6.78 ± 0.06). Female (n=5): pronotum length 9.00–10.24 (9.60 ± 0.48); fore femur length 11.33–13.55 (12.33 ± 0.84); mid femur length 11.96–13.65 (12.79 ± 0.67); hind femur length 28.53–31.73 (30.15 ± 1.29); hind femur width 6.50–7.60 (7.05 ± 0.40); ovipositor length 11.74–13.95 (12.56 ± 0.82).
Etymology. From Latin “stephano” meaning crown and “soltis” referring to the Soltis Center for Research and Education, the type locality of the species. Therefore, stephanosoltis means “Crown of Soltis” referring to the first king cricket ever described at the facility.
Distribution. Costa Rica: Alajuela Province, San Ramón. Forest floor in mid-elevation (450 m above sea level) secondary rainforest.
Holotype: Male ( Fig. 3 View FIGURE 3 ). (Measurement: pronotum length 9.34 mm; fore femur length 11.99 mm; mid femur length 12.09 mm; hind femur length 29.36 mm; hind femur width 6.81 mm.) COSTA RICA: Alajuela Province, San Ramón, San Juan de Peñas Blancas. Soltis Center for Research and Education , 10°23’0.4524’’N, 84°37’4.674’’W, 7.viii.2018, collected by hand at night. Coll. S.J. Richardson. GoogleMaps
Additional Type Material. 26 paratypes (5 adult males, 13 adult females, 5 nymphal males, 3 nymphal females). Same data as holotype.
Type Depository. All type material has been deposited to the Texas A&M University Insect Collection (TAMU- IC).
DNA Barcode. We have generated a DNA barcode for G. stephanosoltis sp. nov., which has been deposited to GenBank with accession number MN 128722 View Materials , and the DNA tissue voucher specimen was deposited to TAMUIC Insect Genomic Collection with voucher number TAMUIC-IGC-002678. Although Vandergast et al. (2017) generated COI genes for many Anostostomatidae , including several unidentified specimens of Glaphyrosoma from Mexico, the primers that they used (C1-J-2183 and C1-N-2872) amplified the back half of the COI gene and did not overlap with the DNA barcode region, which is located in the front half of the COI gene. Therefore, our DNA barcode represents the first for the genus.
Biological Information. Glaphyrosoma stephanosoltis sp. nov. was hand collected along the trails adjacent to secondary rainforest in the vicinity of the Soltis Center for Research and Education. The Soltis Center’s forests are adjacent to the Children’s Eternal Rainforest (Bosque Eterno de los Niños) and located about 450 m above sea level. The insects were only found after sunset when it was sprinkling or after it had just rained, and were more abundant when temperature was above 21 oC. The species was never observed during the day, and when kept in a screen cage with foliage comprised of torn leaves and branches, it was observed that the insects still hid under foliage, even in a dark environment. Those that were unable to hide under the foliage started to appear dull in their cuticles, which suggested that they desiccate easily even on the rainforest floor. The species most likely lives underground in order to avoid desiccation and remain in a higher level of humidity beneath the rainforest floor and only comes out when humidity levels are higher to search for food or mates. When collecting specimens along trails, it was found they tended to hide on the overhanging dirt underside along trails and sometimes in tunnels most likely made by other burrowing animals. When baited with oatmeal, the insects were attracted within an hour of placing the bait, which suggested that these insects might be targeting a good source of carbohydrate and protein, which are rich in the oatmeal used for the trail. When presented with a choice between a plant mixture ( Passiflora sp., Cecropia obtusifolia , Neurolaena lobata , and unidentified Piperaceae ) and a protein mixture (land crab and katydids), all of which were commonly found in the forest, G. stephanosoltis sp. nov. showed overwhelming preference for the plants. However, when kept in a cage with other insects, they fed on dead crickets and katydids, suggesting a necrophagous behavior. Collectively, these observations suggest that G. stephanosoltis sp. nov. is an omnivorous insect, which is a common dietary pattern in many ensiferans.
| MN |
Museu Nacional, Universidade Federal do Rio de Janeiro |
| TAMUIC |
Texas A&M University Insect Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Tribe |
Glaphyrosomatini |
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Tribe |
Glaphyrosomatini |