Phascolarctomorphia Aplin and Archer, 1987
|
publication ID |
https://doi.org/10.1206/0003-0090.457.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.6974221 |
|
persistent identifier |
https://treatment.plazi.org/id/03EFDD5D-F6DF-68CF-D8B5-FCAD1B33FB1D |
|
treatment provided by |
Felipe (2022-08-07 14:35:17, last updated 2024-11-26 19:59:02) |
|
scientific name |
Phascolarctomorphia Aplin and Archer, 1987 |
| status |
|
Phascolarctomorphia Aplin and Archer, 1987
CONTENTS: † Litokoala , † Nimiokoala , and Phascolarctos (fig. 44).
STEM AGE: 32.4 Mya (95% HPD: 29.1–36.4 Mya).
CROWN AGE: 25.3 Mya (95% HPD: 19.6–30.3 Mya).
UNAMBIGUOUS CRANIODENTAL SYNAPOMORPHIES: Extracranial course of mandibular nerve traverses a bony canal in the roof of the hypotympanic sinus (char. 52: 1→2; ci = 0.231); postgenoid vein emerges from the postglenoid foramen in the posteromedial corner of the glenoid fossa, medial or anteromedial to the postglenoid process (char. 77: 0→1; ci = 0.250); and additional cuspid labial to m1 protoconid present, forming a cusplike protostylid (char. 165: 0→1; ci = 0.286).
COMMENTS: Phascolarctidae is consistently recovered in our molecular, morphological and total-evidence analyses ( figs. 27–33 View FIG View FIG View FIG View FIG View FIG View FIG ) as sister to the remaining vombatiforms, which collectively comprise Vombatomorphia (note that we consider † Thylacoleonidae to be Diprotodontia incertae sedis and not a member of Vombatiformes; see above). Aplin and Archer (1987) placed Phascolarctidae in its own infraorder, Phascolarctomorphia (coordinate to Vombatomorphia), and it remains the only known phascolarctomorphian family; thus, the craniodental synapomorphies of Phascolarctidae apply equally to Phascolarctomorphia.
Known phascolarctids are craniodentally distinctive ( Sonntag, 1922; Archer, 1984a, 1984c; Aplin, 1987, 1990; Lee and Carrick, 1989; Springer and Woodburne, 1989; Black and Archer, 1997b; Louys et al., 2009; Black et al., 2014a), and monophyly of this clade is supported by three unambiguous craniodental synapomorphies in our analysis, although all show some degree of homoplasy. Perhaps the most striking of these is the extracranial course of the mandibular nerve, which traverses a bony canal in the roof of the hypotympanic sinus in all three of our phascolarctid terminals († Litokoala , † Nimiokoala and Phascolarctos ; Aplin, 1987; 1990; Louys et al., 2009), a feature that (as far as we are aware) occurs in no other metatherians. 34
In contrast to Black et al. (2012a), we found † Nimiokoala , rather than † Litokoala , to be more closely related to Phascolarctos , with the † Nimiokoala + Phascolarctos clade supported by a single unambiguous synapomorphy (see file S 3 in the online supplement): maxillary and frontal bones in contact on medial orbital wall (char. 13 0→1; ci = 0.143). Like other vombatiform families, the oldest record of Phascolarctidae is from late Oligocene sites in Australia ( Archer et al., 1999; Long et al., 2002; Archer and Hand, 2006; Black et al., 2012b, 2014b).
Aplin, K. P., and M. Archer. 1987. Recent advances in marsupial systematics with a new syncretic classification. In M. Archer (editor), Possums and opossums: studies in evolution: xv - lxxii. Sydney: Surrey Beatty and Sons.
Aplin, K. P. 1987. Basicranial anatomy of the early Miocene diprotodontian Wynyardia bassiana (Marsupialia: Wynyardiidae) and its implications for wynyardiid phylogeny and classification. In M. Archer (editor), Possums and opossums: studies in evolution: 369 - 391. Sydney: Surrey Beatty and Sons.
Aplin, K. P. 1990. Basicranial regions of diprotodontian marsupials: anatomy, ontogeny and phylogeny. Ph. D. dissertation, School of Biological Science, University of New South Wales, Sydney.
Archer, M. 1984 a. On the importance of being a koala. In M. Archer and G. Clayton (editors), Vertebrate zoogeography and evolution in Australasia: 809 - 815. Perth: Hesperian Press.
Archer, M. 1984 c. The Australian marsupial radiation. In M. Archer and G. Clayton (editors), Vertebrate zoogeography and evolution in Australasia: 633 - 808. Perth: Hesperian Press.
Archer, M., and S. J. Hand. 2006. The Australian marsupial radiation. In J. R. Merrick, M. Archer, G. M. Hickey, and M. S. Y. Lee (editors), Evolution and biogeography of Australasian vertebrates: 575 - 646. Sydney: Auscipub Pty Ltd.
Bassarova, M., and M. Archer. 1999. Living and extinct pseudocheirids (Marsupialia, Pseudocheiridae): Phylogenetic relationships and changes in diversity through time. Australian Mammalogy 21: 25 - 27.
Black, K. H., and M. Archer. 1997 b. Nimiokoala gen. nov. (Marsupialia, Phascolarctidae) from Riversleigh, northwestern Queensland, with a revision of Litokoala. Memoirs of the Queensland Museum 41 (2): 209 - 228.
Black, K. H., M. Archer, and S. J. Hand. 2012 a. New Tertiary koala (Marsupialia, Phascolarctidae) from Riversleigh, Australia, with a revision of phascolarctid phylogenetics, paleoecology, and paleobiodiversity. Journal of Vertebrate Paleontology 32 (1): 125 - 138.
Black, K. H., M. Archer, S. J. Hand, and H. Godthelp. 2012 b. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In J. A. Talent (editor), Earth and life: global biodiversity, extinction intervals and biogeographic perturbations through time: 983 - 1078. Dordrecht: Springer Verlag.
Black, K. H., J. Louys, and G. J. Price. 2014 a. Understanding morphological variation in the extant koala as a framework for identification of species boundaries in extinct koalas (Phascolarctidae; Marsupialia). Journal of Systematic Palaeontology 12 (2): 237 - 264.
Black, K. H., G. J. Price, M. Archer, and S. J. Hand. 2014 b. Bearing up well? Understanding the past, present and future of Australia's koalas. Gondwana Research 25 (3): 1186 - 1201.
Lee, A. K., and F. N. Carrick. 1989. 31. Phascolarctidae. In D. W. Walton and B. J. Richardson (editors), Fauna of Australia, vol. 1 B. Mammalia: 1 - 31. Canberra: AGPS.
Long, J. A., M. Archer, T. F. Flannery, and S. J. Hand. 2002. Prehistoric mammals of Australia and New Guinea: one hundred million years of evolution, Sydney: UNSW Press.
Louys, J., K. P. Aplin, R. M. D. Beck, and M. Archer. 2009. Cranial anatomy of Oligo-Miocene koalas (Diprotodontia: Phascolarctidae): stages in the evolution of an extreme leaf-eating specialization. Journal of Vertebrate Paleontology 29 (4): 981 - 992.
Sonntag, C. F. 1922. On the myology and classification of the wombat, koala, and phalangers. Proceedings of the Zoological Society of London 92 (4): 863 - 896.
Springer, M. S., and M. O. Woodburne. 1989. The distribution of some basicranial characters within the Marsupialia and a phylogeny of the Phalangeriformes. Journal of Vertebrate Paleontology 9 (2): 210 - 221.
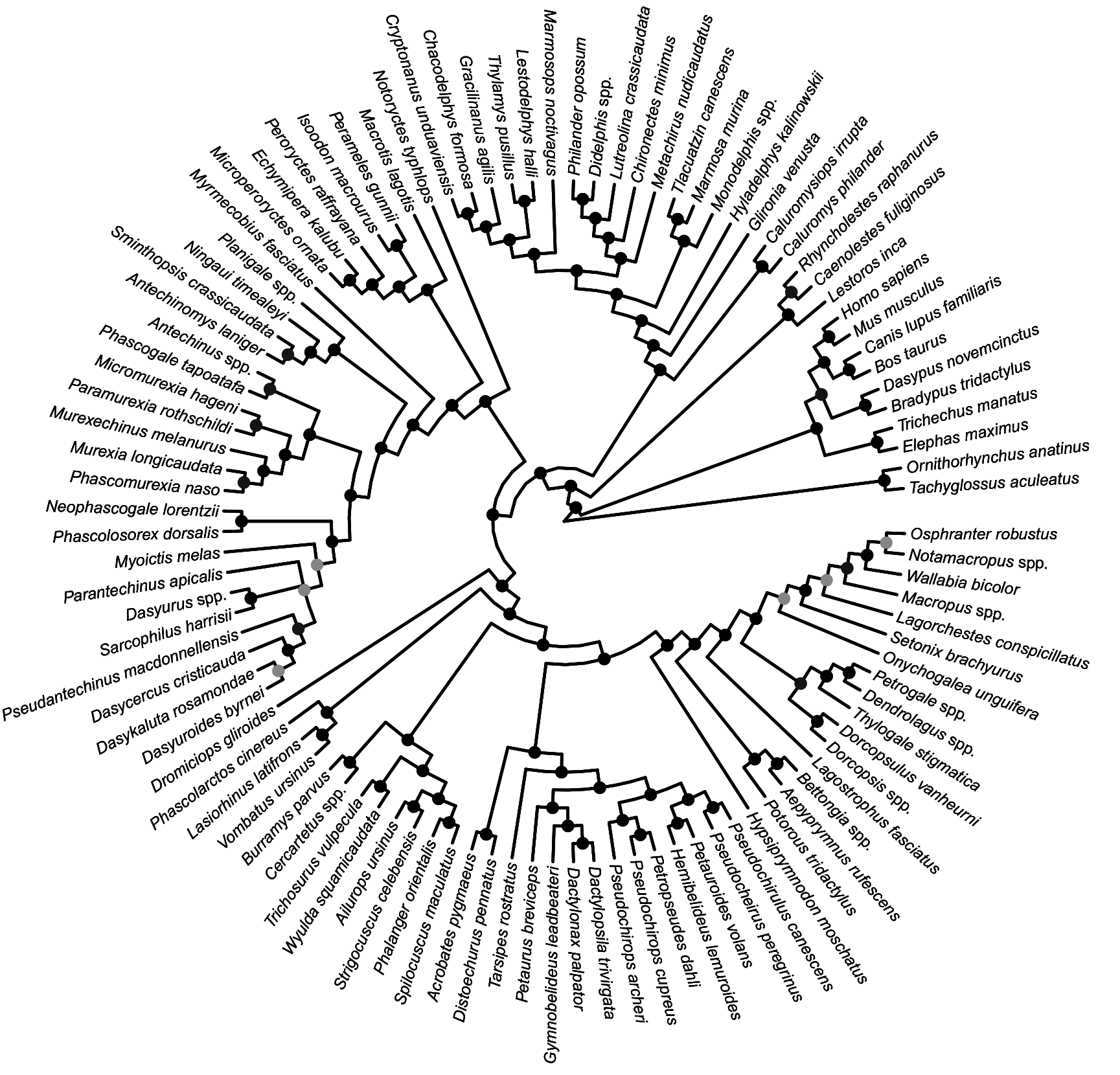
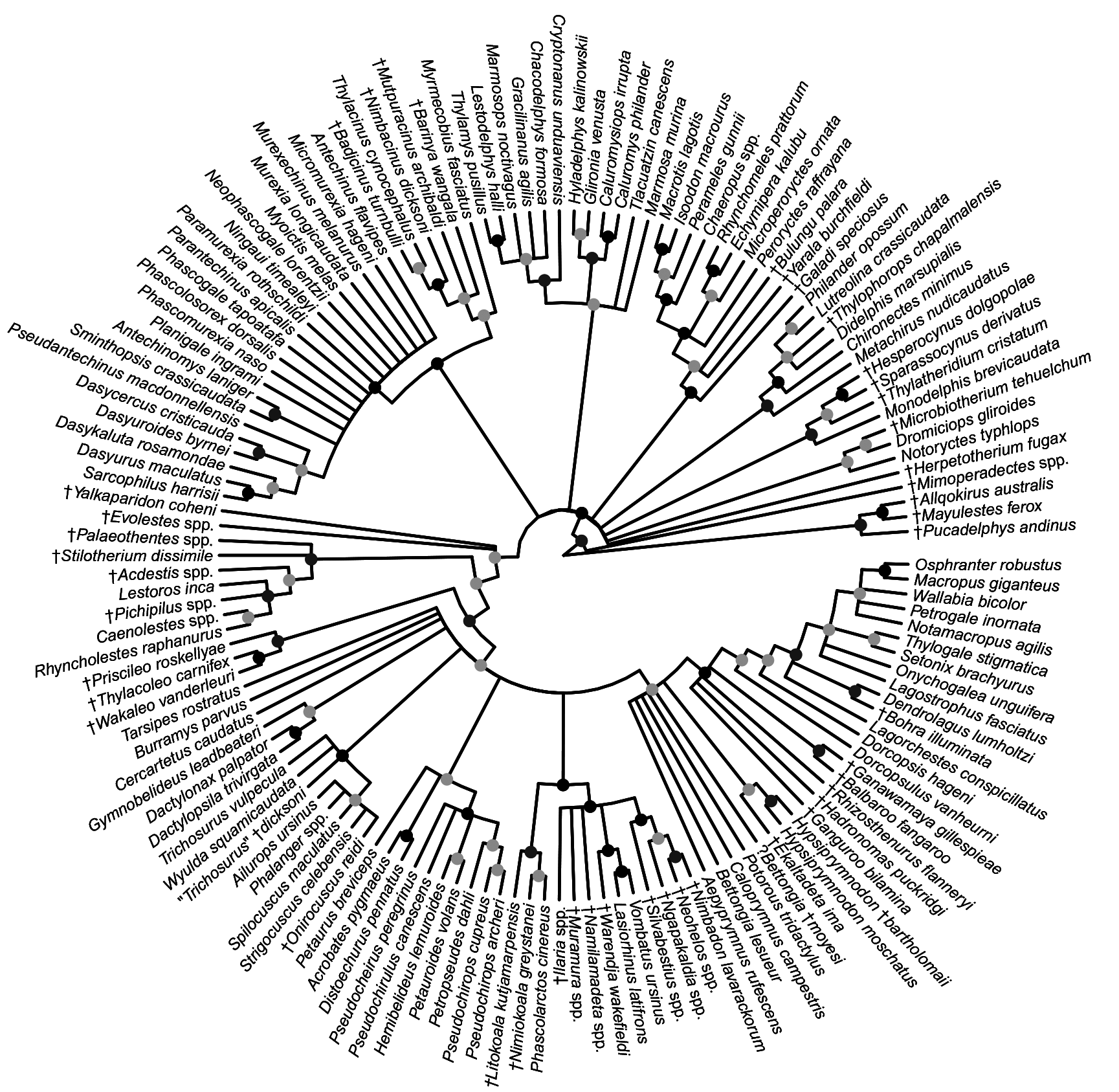
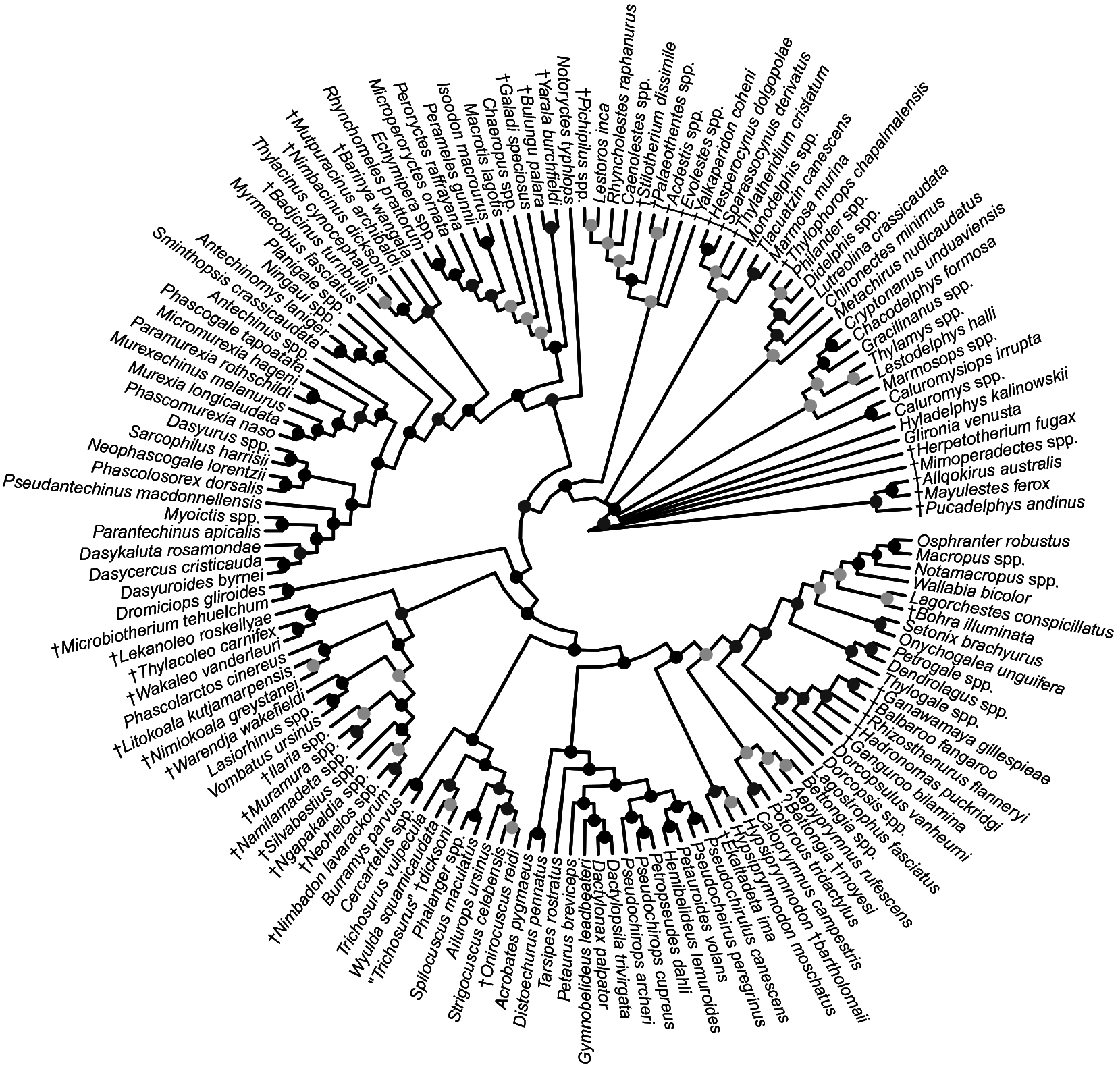
FIG. 27. Fifty-percent majority rule consensus of post-burn-in trees that results from Bayesian analysis of our nuclear sequence-only dataset. Black dots at nodes indicate ≥0.95 Bayesian posterior probability (“strong support”); dark gray dots indicate 0.75–0.94 Bayesian posterior probability (“moderate support”); light gray dots indicate 0.50–0.74 Bayesian posterior probability (“weak support”).
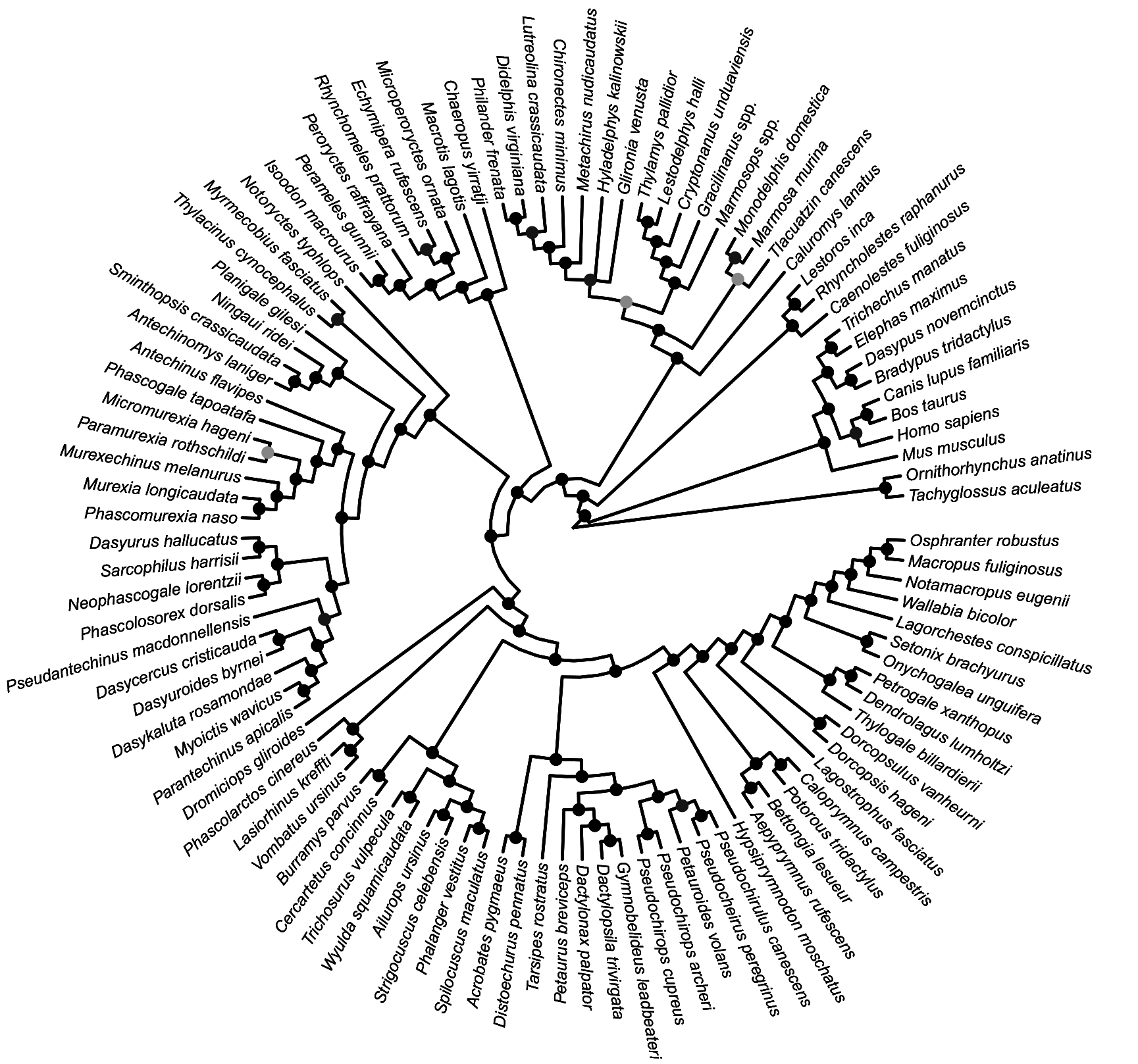
FIG. 28. Fifty-percent majority rule consensus of post-burn-in trees that results from undated Bayesian analysis of our mitochondrial sequence-only dataset. Black dots at nodes indicate ≥0.95 Bayesian posterior probability (“strong support”); dark gray dots indicate 0.75–0.94 Bayesian posterior probability (“moderate support”); light gray dots indicate 0.50–0.74 Bayesian posterior probability (“weak support”).
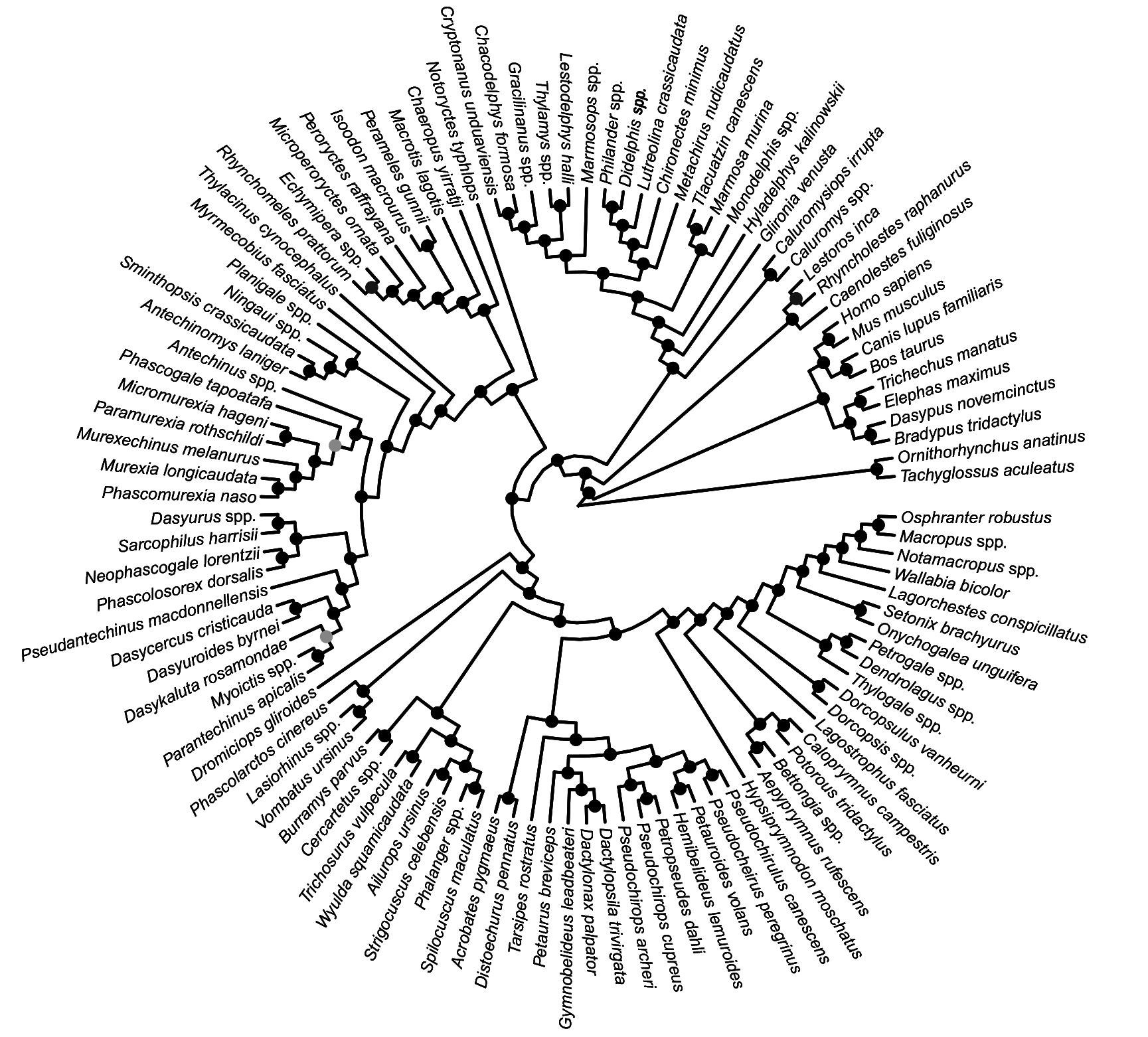
FIG. 29. Fifty-percent majority rule consensus of post-burn-in trees that results from undated Bayesian analysis of our combined nuclear and mitochondrial sequence dataset. Black dots at nodes indicate ≥0.95 Bayesian posterior probability (“strong support”); dark gray dots indicate 0.75–0.94 Bayesian posterior probability (“moderate support”); light gray dots indicate 0.50–0.74 Bayesian posterior probability (“weak support”).
FIG. 30. Strict consensus of 99,999 most parsimonious trees (each of 949 steps, consistency index =0.269, retention index = 0.815) that result from maximum parsimony analysis of our morphological dataset. Black dots at nodes indicate ≥70% bootstrap support (“strong support”); dark gray dots indicate 50%–69% bootstrap support (“moderate support”); light gray dots indicate <50 bootstrap support (“weak support”).
FIG. 31. Fifty-percent majority rule consensus of post-burn-in trees that results from undated Bayesian analysis of our morphological dataset. Black dots at nodes indicate ≥0.95 Bayesian posterior probability (“strong support”); dark gray dots indicate 0.75–0.94 Bayesian posterior probability (“moderate support”); light gray dots indicate 0.50–0.74 Bayesian posterior probability (“weak support”).
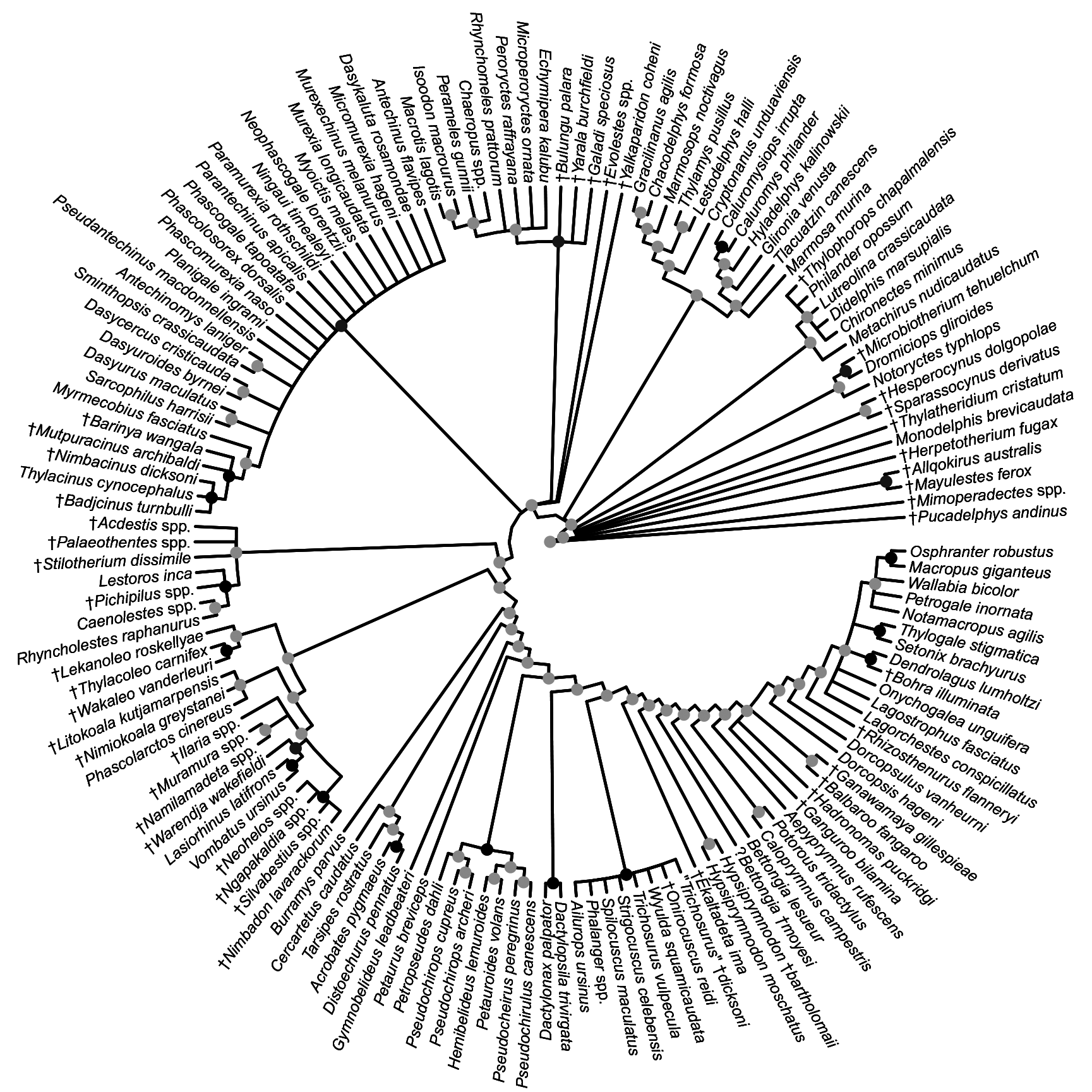
FIG. 32. Fifty-percent majority rule consensus of post-burn-in trees that results from undated Bayesian analysis of our total evidence dataset. Black dots at nodes indicate ≥0.95 Bayesian posterior probability (“strong support”); dark gray dots indicate 0.75–0.94 Bayesian posterior probability (“moderate support”); light gray dots indicate 0.50–0.74 Bayesian posterior probability (“weak support”).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
1 (by felipe, 2022-08-07 14:35:17)
2 (by felipe, 2022-08-08 13:46:01)
3 (by felipe, 2022-08-08 14:10:54)
4 (by felipe, 2022-08-08 14:49:10)
5 (by felipe, 2022-08-08 15:08:29)
6 (by felipe, 2022-08-08 15:15:06)
7 (by felipe, 2022-08-08 17:31:39)
8 (by ExternalLinkService, 2022-08-08 17:40:02)
9 (by ExternalLinkService, 2022-08-08 19:30:07)
10 (by ExternalLinkService, 2022-08-30 20:38:21)
11 (by ExternalLinkService, 2022-08-30 20:38:21)