Hyalinobatrachium dianae, Kubicki, Brian, Salazar, Stanley & Puschendorf, Robert, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3920.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:D03CFD04-F9BA-47E9-8DBD-920C539FF4F0 |
|
DOI |
https://doi.org/10.5281/zenodo.6102996 |
|
persistent identifier |
https://treatment.plazi.org/id/03F29955-FFDD-FF9B-9382-6E5AFE24D6E0 |
|
treatment provided by |
Plazi |
|
scientific name |
Hyalinobatrachium dianae |
| status |
sp. nov. |
Hyalinobatrachium dianae View in CoL sp. nov.
Diane’s bare-hearted glassfrog / rana de vidrio de Diane ( Fig. 1 View FIGURE 1 )
Holotype. UCR 22038, an adult male from Costa Rica: Provincia de Heredia: Cantón de Sarapiquí: Distrito de Horquetas: approximately 4km west of Santa Clara, ca. 400 m a.s.l. (N 10.219, W 83.949), obtained by Brian Kubicki and Stanley Salazar on 13 October 2013.
Paratopotypes. UCR 22037, adult female, same data as the holotype; UCR 22034, adult male, same data as the holotype but obtained by Stanley Salazar on 18 November 2013.
Paratypes. UCR 22033, an adult male from Costa Rica: Provincia de Limón: Cantón de Turrialba: Distrito de Chirripo: headwaters of Quebrada Surubre, ca. 900 m a.s.l. (N 9.973, W 83.392), obtained by Norberto Solano on 1 August 2012. UCR 22035–36, two adult males from Costa Rica: Provincia Limón: Cantón de Limón: Distrito Río Blanco: head waters of Río Victoria, ca. 400 m a.s.l. (N 9.915, W 83.188), obtained by Stanley Salazar on 22 November 2013.
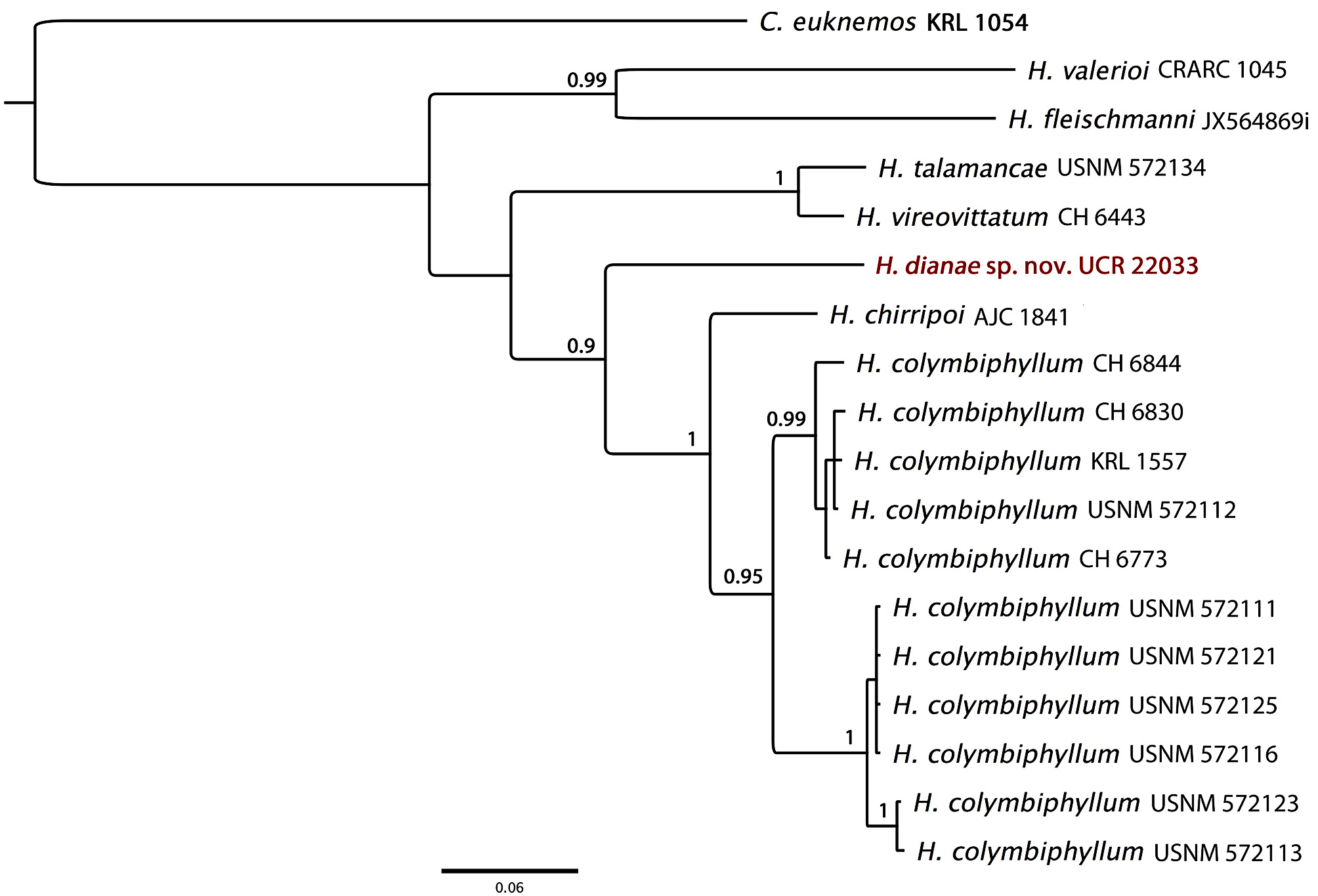
Generic Placement. We assign this new species to the genus Hyalinobatrachium due to the combination of the following characteristics that are outlined as diagnostics for members of this genus ( Guayasamin et al. 2009): (1) humeral spine absent in adult males; (2) digestive tract and bulbous liver covered in white iridophores; (3) parietal peritoneum transparent, allowing viscera to be fully visible when viewed ventrally; (4) white bones in life; (5) dorsal coloration in preservative cream; (6) lacking vomerine teeth and dentigerous process of the vomer; (7) adult males typically seen calling from the underside of the foliage; (8) egg masses deposited in a single layer on the inferior surface of leaves.
Diagnosis. Herein we follow the standardized character states for diagnosing centrolenids presented by Cisneros-Heredia & McDiarmid (2007). The combination of the following characteristics can be used to distinguish Hyalinobatrachium dianae from other members of the family Centrolenidae : (1) lack of vomerine teeth and dentigerous process of the vomer; (2) snout truncate in dorsal and lateral views; (3) tympanic membrane and tympanic annulus indistinct, covered in skin and indiscernible in color and texture from adjacent surfaces; (4) skin on dorsal surfaces of limbs, body and head granular, slightly increased granular texture on body ( Fig. 2 View FIGURE 2 A); (5) ventral skin of thighs and body slightly granular, with most pronounced granulation on abdomen; chin and ventral surfaces of the arms and lower legs are smooth; subcloacal skin containing a concentration of weakly enameled smooth tubercles, lacking large subcloacal tubercles or folds; (6) parietal and cardial peritonea transparent; urinary bladder transparent; hepatic and gastrointestinal peritonea containing iridophores; gallbladder transparent yellowish green in living specimens ( Fig. 2 View FIGURE 2 B); (7) liver bulbous; (8) adult males lack humeral projection; (9) lacking webbing between fingers I and II, basal webbing present between fingers II and III, moderate webbing between fingers III and IV, III 2 -–2+ IV ( Fig. 3 View FIGURE 3 A); (10) toe webbing I 1 3/4–2 II 1 1/4–2+ III 1 1/2–2 3/4 IV 2 1/2–1 1/ 2 V ( Fig. 3 View FIGURE 3 B); (11) a very weak white fleshy fringe extends from the elbow along the ventrolateral margin of forearm and Finger IV to the base of the disc; a very weak white fleshy fringe is present on the lower leg, originating at the heel and extending along the ventrolateral margin of tarsus, metatarsus, and to the base of Toe V; (12) nuptial excrescence (type V) present as a small cluster of minute glandular granules on the basal to medial dorsolateral edge of Finger I; prepollex not enlarged and prepollical spine not protruding; (13) Finger I longer than Finger II when measured from the corresponding distal margin of the palmar tubercle to the digit tip; (14) diameter of eye roughly twice the width of disc on Finger III; (15) living individuals have uniform lime green dorsal surfaces, lacking any evident light or dark spots; a dark oblong spherical structure under the skin of the lower back; bones white in life; (16) preserved specimens have a uniform cream-yellow dorsal coloration, with numerous minute dark star-like melanophores present throughout; bones white in preserved specimens; (17) iris coloration in life: silverywhite with fine dark spots or reticulation that become more concentrated directly surrounding the horizontally elliptical pupil; iris coloration in preservative: similar to that in life with a silvery white background and contrasting dark purplish spots or reticulation; (18) digits of hands and feet uniformly yellow, with the exception of the dorsal surfaces of toes IV and V which have a mixture of pigmention typical of the adjacent dorsal surface of the metatarsus; melanophores absent on the digits of hands and toes I, II, and III; (19) males have been observed calling from the undersurfaces of leaves; advertisement call is a single tonal note, a long metallic whistle-like note with a very rapid but weakly pulsed intensity; note duration 0.40– 0.55 s (average 0.501 s), dominant frequency 3.35–3.44 kHz (average 3.39 kHz) (N=9); (20) egg masses have been observed on the underside of the leaves, in a single layer; clutch size 31– 68 eggs (N = 9); early-staged embryos are greenish-yellow; males have been observed attending eggs during the night; (21) SVL in adult males 28.5–29.4 mm (N = 5), adult female 28.0 mm (N = 1). Combat behavior and tadpoles are unknown for the new species.
Comparisons. Due to their small size, potential inter/intrapopulational variation, and general lack of evident morphological characters, especially when examining preserved specimens, some members of the genus Hyalinobatrachium present difficulty when it comes to making interspecific comparisons based solely on their morphology. This difficulty in discerning some Hyalinobatrachium species based solely on morphological characters has been noted by previous authors as well, and resulted in their use of an integrative taxonomy approach that employs a combination of morphological characters, advertisement calls, and phylogenetic relationships to define the different species addressed in their taxonomic studies (Castroviejo-Fisher et al. 2009; 2011; Twomey et al. 2014). Herein we will also be utilizing an integrative comparative approach where we look at aspects involving morphological and bioacoustic characteristics, in addition to COI DNA sequences. Being that the new species is only known to occur in Costa Rica, our comparisons are narrowed down to congeners native to Central America, the Magdalena and Cauca river valleys of Colombia, and the Chocó biogeographical region of western Colombia and Ecuador. Additionally, being that our studies have shown H. dianae to have the closest relation to H. chirripoi and H. colymbiphyllum we have included in our comparisons two species that are not native to the above-mentioned regions. The two additional species that we included morphological and bioacustic comparisons are H. anachoretus and H. pellucidum , both of which occur east of the Andes and were shown to have a close relation to both H. chirripoi and H. colymbiphyllum according to the phylogeny presented by Twomey et al. (2014). We did not include any genetic comparison to H. anachoretus and H. pellucidum being that COI sequences are not available for these two species.
Contrasting characteristics for H. dianae are presented in parentheses. Hyalinobatrachium anachoretus Twomey et al., 2014 has yellow spots on the dorsum (lacking yellow spots); advertisement call lasting 0.32– 0.37 s, containing 5–6 distinct pulses, and having a dominant frequency of 4.67–4.8 kHz (total duration of 0.40– 0.55 s [average 0.501 s]), a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium aureoguttatum ( Barrera-Rodríguez & Ruiz-Carranza, 1989) has distinct large yellow spots on the dorsum that become white in preservative (lacking yellow spots); the advertisement call of H. aureoguttatum is unavailable for comparison. Hyalinobatrachium chirripoi ( Taylor, 1958) has substantial webbing between fingers II–III and III–IV ( Kubicki 2004; 2007) (substantial webbing being found only between fingers III–IV), light yellow spots in the dorsal skin (lacking any light yellow spots in the dorsal skin, Fig. 4 View FIGURE 4 ), the advertisement call of H. chirripoi is a rapid high-pitched trill, comprised of numerous individual pulses, a single examined advertisement call of a male H. chirripoi from a tributary of Río Banano, Limón Province, Costa Rica, 80 m a.s.l. had a total duration of 0.285 seconds, a dominant frequency of 4.3 kHz, and 18 individual pulses (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium colymbiphyllum ( Taylor, 1949) has light yellow spots in the dorsal skin (lacking any light yellow spots in the dorsal skin, Fig. 4 View FIGURE 4 ), the advertisement call of H. colymbiphyllum is a high-pitched trill comprised of numerous distinct pulses, single advertisement calls from two males of H. colymbiphyllum from two different locations within Costa Rica were examined for this study. The first call is from a male near the Santa Elena Reserve in Tilarán, Guanacaste Province, 1550 masl. This call had a total duration of 0.628 seconds, a dominant frequency of 4.0 kHz, and 13 distinct pulses. The second H. colymbiphyllum advertisement call is from a male near Rincón de Osa, Puntarenas Province, 40 masl. This call had a total duration of 0.32 seconds, a dominant frequency of 4.6 kHz, and 9 distinct pulses (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium fleischmanni ( Boettger, 1893) differs by having light yellow spots in the dorsal skin, and a pericardial sac containing iridophores, (lacking light yellow spots in the dorsal skin, and having a transparent pericardial sac); the advertisement call of H. fleischmanni differs from that of H. dianae by being a rapid highpitched rising whistle. A single examined advertisement call of H. fleischmanni from a small stream near Santa Elena, Herédia Province, Costa Rica, 1400 masl, which is located approximately 10 km straight line distance from the type locality for H. fleischmanni of San José, Costa Rica, had a total duration of 0.17 seconds, a dominant frequency of 4.1 kHz, and lacking a pulsed intensity (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium pellucidum ( Lynch & Duellman, 1973) , according to Wen et al. (2012) has a single tonal note advertisement call, with each note lasting 0.12– 0.15 s in duration, and having a dominate frequency of 4.86–5.41 kHz (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium talamancae ( Taylor, 1952) has light yellow spots in the dorsal skin, and a distinct green mid-dorsal stripe ( Kubicki 2006; 2007) (lacking both light yellow spots in the dorsal skin and a distinct green mid-dorsal line); Hyalinobatrachium talamancae produces a long whistle-like advertisement call, consisting of a single non-pulsed note. A single examined advertisement call of H. talamancae from a small stream near Guayacán de Siquirres, Limón Province, Costa Rica, 500 masl, had a total duration of 0.3 s, and a dominant frequency of 4.9 kHz (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium valerioi ( Dunn, 1931) has a green reticulation of the dorsal skin, and a fully to partially white pigmented pericardial sac (lacking a green reticulation of the dorsal skin, and pericardial sac completely lacking white iridophores); Hyalinobatrachium valerioi produces a short “peep” advertisement call. A single examined advertisement call of H. valerioi from a small stream near Guayacán de Siquirres, Limón Province, Costa Rica, 300 m a.s.l., had a total duration of 0.05 s, and a dominant frequency of 7.5 kHz (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]). Hyalinobatrachium vireovittatum ( Starrett & Savage, 1973) has light yellow spots in the dorsal skin, and a distinct green mid-dorsal line bordered on both sides by yellow paravertebral stripes (lacking both light yellow spots in the dorsal skin and a distinct green mid-dorsal line bordered on both sides by yellow paravertebral stripes); Hyalinobatrachium vireovittatum produces a long whistle-like advertisement call, consisting of a single non-pulsed note. A single examined advertisement call of H. vireovittatum from a small stream northwest of Palmar Norte, Puntarenas Province, Costa Rica, 550 masl, had a total duration of 0.264 s, and a dominant frequency of 4.6 kHz (total duration of 0.40– 0.55 s [average 0.501 s], a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity, lacking distinct pulses, and a dominant frequency of 3.35–3.44 kHz [average 3.39 kHz]).
Description of holotype. Adult male having a SVL of 28.5 mm. Head slightly wider than body, with greatest width just posterior to the articulation of the jaws; upper lip round in dorsal outline, not flared; snout raised anterodorsally, truncate in dorsal outline and profile; snout short with nearly terminal protuberant nostrils directed laterally; internarial area concave. Eyes large and directed forward. Top of head flat; canthus rostralis distinct; intercanthal area slightly concave, loreal region slightly concave. Skin on all dorsal and lateral surfaces of head moderately granular; tympanic membrane and tympanic annulus indistinct, covered in skin. A weak but discernible supratympanic fold is present. Upper surfaces of body and limbs granular. Flanks smooth to weakly granular, especially along the anterior flank region; skin on the chin smooth. Skin of the chest, ventral surfaces of the body, and thighs weakly granular; skin of the groin and ventral surfaces of the arms and lower legs nearly smooth.
Arms short, with forearm slightly enlarged; humeral spine absent; no distinct transverse dermal fold on wrist; a very weak white fleshy fringe extends from the elbow along the ventrolateral margin of forearm, metacarpus, and Finger IV to the base of the disc. Hands are large (HaL 8.9 mm, 31.2% of SVL); fingers moderately long and robust with weak lateral fringes; discs on fingers truncate. Discs on fingers I, III, and IV nearly equal in width; disc on Finger I (1.6 mm) minutely wider than discs on fingers III and IV; disc on Finger II narrowest (1.3 mm). Nuptial excrescence (type V), which is present as a small cluster of minute glandular granules on the basal to medial dorsolateral edge of Finger I; prepollex not enlarged and prepollical spine not protruding. Subarticular tubercles on fingers III and IV indistinct. Subarticular tubercles on fingers I and II distinct, round and slightly raised; supernumerary tubercles on the proximal side of subarticular tubercles on fingers I and II distinct, round and slightly raised. Other supernumerary tubercles indistinct; accessory palmar tubercles very weak to indistinct; thenar and palmar tubercles poorly developed, thenar ovoid and palmar round. Webbing absent between fingers I–II and basal between fingers II–III; moderate webbing found between fingers III–IV, reaching the proximal margin of the distal subarticular tubercle on Finger III and the distal margin of the subarticular tubercle on Finger IV. Webbing formula for between fingers III and IV: III 2 -–2+ IV. Finger lengths radiating from corresponding margin of palmar tubercle to the digit tip: Finger I (4.9 mm), Finger II (4.1 mm), Finger III (6.9 mm), and Finger IV (5.9 mm); relative lengths of fingers II <I <IV <III.
Legs relatively long and thin; tibiotarsal articulation extending well beyond tip of snout when hind limb adpressed; a very weak white fleshy fringe is present, originating on the heel and extending along ventrolateral margin of tarsus, metatarsus, and to base of Toe V; discs on toes slightly rounded to somewhat truncate; narrower than those on fingers, widest on toes IV and V (Toe I 0.7 mm, Toe II 0.9 mm, Toe III 1.0 mm, Toe IV 1.3 mm, Toe V 1.2 mm); subarticular tubercles under the toes small, round and slightly elevated; inner metatarsal tubercle elongate, slightly raised, outer metatarsal tubercle not well distinguished from the numerous small accessory tubercles; slight inner tarsal fold present; toes moderately webbed, webbing extending to penultimate phalanges on one margin on four toes; web margin slightly concave; toes webbing formula: I 1 3/4–2 II 1 1/4–2+ III 1 1/2–2 3/4 IV 2 1/2–1 1/ 2 V. Cloacal opening directed posteriorly at mid-level of thighs with a concentration of small weakly enameled (white) tubercles below cloaca.
Tongue round in shape, lacking a distinct posterior notch; prevomerine dentigerous processes and teeth absent; choanae moderately large, rounded on posterior half, but flat on anterior half, hemispherical; paired elongate vocal slits present, extending from posterolateral base of tongue towards the angle of the jaw; vocal sac single, median, subgular.
A dark oblong spherical structure is found under the skin of the lower back, to left of the center axis of the body in the pelvic region ( Fig. 5 View FIGURE 5 ). This structure has been observed in all of the specimens of Hyalinobatrachium dianae examined during this study, whether it serves a function is unknown.
Coloration in life. Dorsal background color uniform Lime Green (105), lacking light yellow spots. Granules of dorsal skin often with a slightly lighter shade, varying from Pale Greenish White (97) to Light Yellow Green (87), subject to metachrosis. The hands and feet have a Pale Greenish Yellow (86) coloration, especially concentrated on the digits and webbing. The iris has a white background color with darker pigments in the form of fine to moderate-sized spots, or a even a fine reticulation in some individuals. The dark spotting or reticulation of the iris is Fuscous (283), and it typically becomes more concentrated directly surrounding the horizontally elliptical pupil. Directly below the cloaca there is a concentration of weakly white-pigmented tubercles. The subdermal oblong spherical structure of the dorsal pelvic region is black.
The ventral skin of the body is transparent; parietal peritoneum transparent; parietal pericardium transparent; bones white. The bulbous liver and digestive organs are covered in white peritonea. The heart and ventral vein are blood red. Lungs transparent, but with a network of red blood vessels. The gall bladder is transparent Sulphur Yellow (91).
Coloration in preservative. The green dorsal coloration has faded to a pale cream-yellow. The only noticeable contrasting structures of the dorsum are the dark star-shaped melanophores, and the dark colored oblong spherical structure under the dorsal skin of the pelvic region.
Measurements. Holotype: SVL 27.4 mm; HW 10.7 mm, 37.5 % of SVL; HL 8.6 mm, 30.2% of SVL; TI 17.0 mm, 59.6% of SVL; FL 12.2 mm, 42.8% of SVL; HaL 8.9 mm, 31.2% of SVL; IOD 3.1 mm, 10.9% of SVL; END 2.6 mm, 9.1% of SVL; FoL 6.0 mm, 21.1% of SVL; TaL 9.1 mm, 31.9% of SVL; EL 3.0 mm; DW 1.6 mm, 53.3% of EL.
Variation. The dark oblong spherical structure under the skin of the dorsal pelvic region showed slight variation in its orientation to the center axis of the body, being positioned slight off center to the left or right in the different type specimens.
The female paratopotype had more of an acute shape of the snout in lateral view compared to the truncate snout of the five males of the type series. Being that only one female is known for Hyalinobatrachium dianae , we are unclear if this was simply a case of individual malformation, or if it is possible that this species shows sexual dimorphism in snout profile.
Molecular genetics. Our studies have shown that Hyalinobatrachium dianae is highly divergent from other members of the genus Hyalinobatrachium that have COI sequences available on GenBank ( Fig. 6 View FIGURE 6 ). The conger that presents the closest relationship to H. dianae is H. chirripoi (12.4%). Despite the relatively low number of COI sequences for Hyalinobatrachium on GenBank, a good representation of the species native to Central America are available, lacking only H. aureoguttatum and H. valerioi . We have included a COI sequence of H. valerioi for additional comparison. Being that our analysis only looked at a single gene (COI) we use our data to infer alphalevel differences among those species which have COI sequences available on GenBank, and do not attempt to imply any broader phylogenetic relationships. Lacking a larger sampling of South American Hyalinobatrachium species sequenced for COI makes a phylogenetic assessment of this gene for the genus difficult at this time. One COI mtDNA sequence for both H. dianae (accession number: KJ703103 View Materials ), and H. valerioi (accession number: KM925140 View Materials ) have been deposited in GenBank.
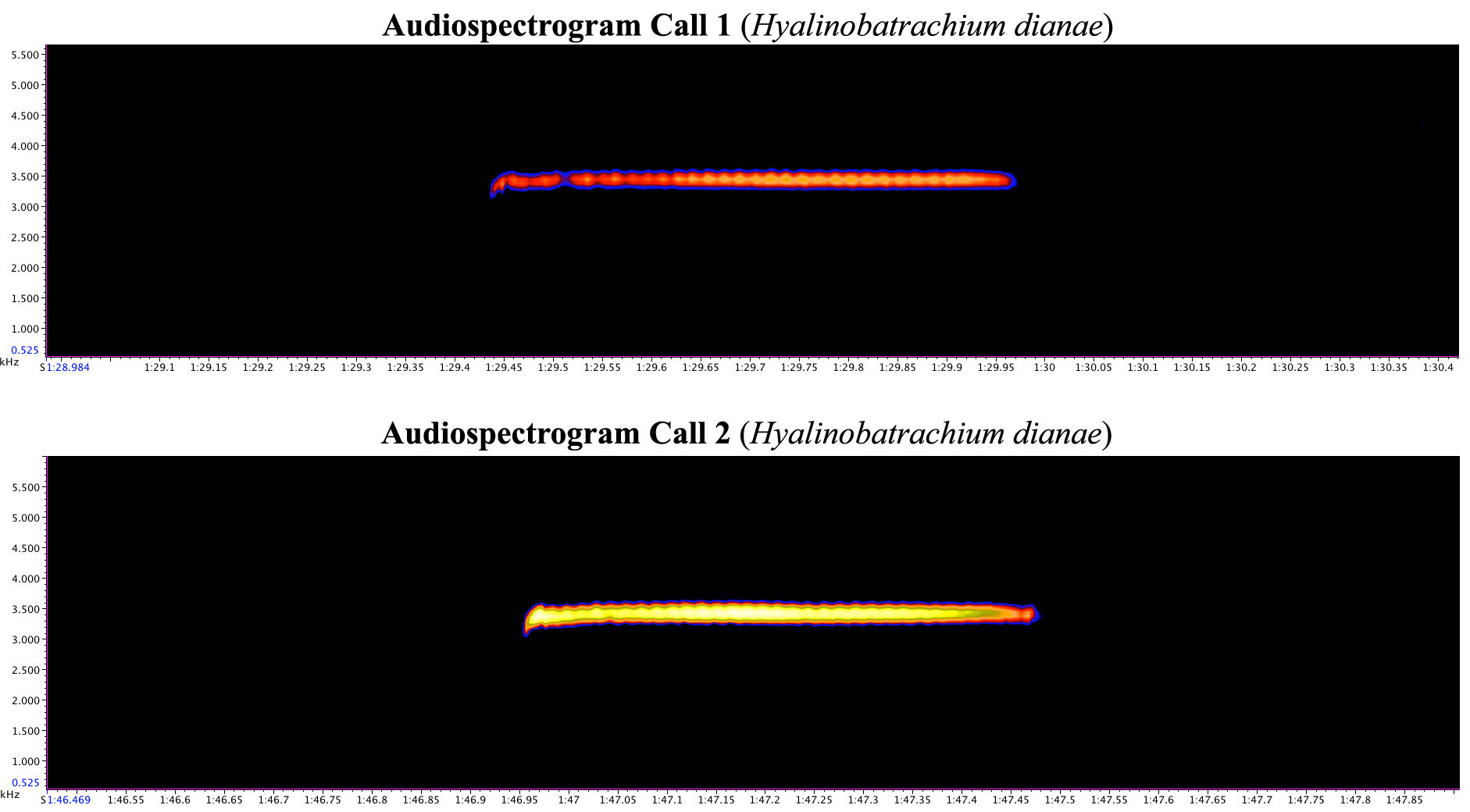
Advertisement call. Males of this species call at night from sites among the forest understory. Males have only been observed calling from the underside of the vegetation, but being that some other congeners can at times be observed calling from the upper leaf surface ( Kubicki 2007), it is possible that Hyalinobatrachium dianae males occasionally call from the upper leaf surfaces as well. Calling males have been observed overhanging the ground instead of moving water like most centrolenids. The advertisement call of H. dianae is quite unique, it consists of a single tonal long metallic whistle-like note with a very rapid but weakly pulsed intensity. Despite having a weakly pulsed intensity, the advertisement calls of H. dianae lacks distinctly discernible pulses like those in H. chirripoi and H. colymbiphyllum .
On 13 October 2013, nine advertisement calls were recorded from the male holotype prior to collection. The male was observed calling alongside a single egg mass, and positioned over a trail with a trickle of water flowing down its path due to recent rain showers; normally this trail is dry without flowing water. The calls were recorded at 20:15–20:30 hours: ambient temperature 22.7 C; relative humidity 95–100%. The advertisement call of Hyalinobatrachium dianae has total duration of 0.40– 0.55 s (average 0.501 s), and a dominant frequency of 3.35–3.44 kHz (average 3.39 kHz). The accompanying audiospectrograms of H. dianae ( Fig. 7 View FIGURE 7 ) represent two calls from the nine that were recorded and measured for this study. Call 1 consisted of a single note, with a total duration of 0.53 s and having a dominant frequency of 3.43 kHz. Call 2 consisted of a single note, with a total duration of 0.52 seconds and having a dominant frequency of 3.4 kHz. The “pulses” of the advertisement call are so close together and weak that it is difficult to determine an exact number from visual examination of the audiospectrograms in Raven Lite 1.0. Our estimates from visual examination of the audiospectrograms included in Figure 7 View FIGURE 7 are 33 weak pulses in call 1, and 30 weak pulses in call 2. Extrapolation from this estimated number of pulses in the two advertisement calls examined would result in approximately 60 pulses per second of note.
Audiospectrograms of related Costa Rican congeners to Hyalinobatrachium dianae are provided for comparison ( Fig. 8 View FIGURE 8 ).
Etymology. We propose the name “ dianae ” in dedication to the senior author’s mother, Janet Diane Kubicki, who always encouraged Brian’s life-long interest with natural history and especially fishes and amphibians. Additionally alluding to the Roman goddess of the hunt, wild animals and woodlands, Diana, who was believed to have a preference of dwelling in sacred forests on high mountains. This being in relation to our own “hunt” among Costa Rica’s mountainous forests to better understand the amphibians dwelling within.
Habitat and ecology. Hyalinobatrachium dianae is a nocturnal frog that has been observed to inhabit mature secondary and primary humid forests with varying topography. This species has not been encountered in high densities, during evenings when we have encountered actively calling males typically only one to three individuals have been heard or observed at a particular site. Egg masses are laid on the underside of the leaf in a single layer ( Fig. 9 View FIGURE 9. A ). In nine masses that were counted, the number of eggs ranged from 31– 68 eggs. Males have been observed at night calling next to the egg masses on the underside of the vegetation between 0.5–5 meters above the ground. Males have also been noted to have the peculiar behavior of calling and/or attending egg masses over apparently dry forest substrate or dry washes instead of overhanging water, as is typical for members of the family Centrolenidae . The sites from which H. dianae has been observed calling and reproducing are located on sloping low areas in relative proximity to streams. It is possible these sites are chosen that in the event of heavy rains the surface runoff water will channel into these sloping low areas and provide flowing water connectivity to a nearby stream for any larvae that hatch. Our field observations (BK & SS) thus far have shown that this species has unpredictable calling activity, with calling males being heard on one night, then on a different night (even with apparently similar climatic and lunar conditions) not a single male can be heard calling.
Distribution. This species is only known from three sites along the Caribbean foothills of Costa Rica, between the vicinity of Santa Clara, Heredia Province and the headwaters of Río Victoria, Limón Province. Hyalinobatrachium dianae has been observed at elevations between 400 to 900 m.a.s.l. ( Fig. 10 View FIGURE 10 ). The known sites for this species are within the life zones of Tropical Wet Forest and Tropical Premontane Rain Forest ( Holdridge 1967). It is likely that this species ranges further to the northwest and to the southeast along the same Caribbean foothills of the Cordillera Volcanica Central and the Cordillera de Talamanca within Costa Rica. It is also possible that the distribution of this species extends on to the Caribbean slopes of northwestern Panama.
Remarks. Most of the known geographic range of Hyalinobatrachium dianae is covered by large intact tracts of premontane rainforests along the Caribbean slopes of the Central Volcanic and Talamancan mountain ranges of Costa Rica where several protected areas exists under the National System of Areas of Conservation (Sistema Nacional de Areas de Conservacion, SINAC). Very few roads grant access to the overall region that H. dianae is known to inhabit, so in the near future we foresee only very limited human threats to this species. How potential infectious disease and climatic change might affect this species in the future is unclear at this moment.
We relate the recent discovery of this species in part to the fact that its known distribution lies in an area that has remained relatively unexplored due to the limited access. Additional factors that may have led to the recent discovery of Hyalinobatrachium dianae include its unusual call, being more similar to an insect than other centrolenids known to inhabit Costa Rica, and our observations that have shown this species exhibits unpredictable calling activity, in which it can be more common to visit a site and not hear calling males than it is to arrive at a site and find calling males.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |