Diutius pallidus Opitz
|
publication ID |
https://doi.org/10.1649/072.068.0313 |
|
persistent identifier |
https://treatment.plazi.org/id/03F3879E-9364-8863-FF48-FE32FE90FE8A |
|
treatment provided by |
Valdenar |
|
scientific name |
Diutius pallidus Opitz |
| status |
sp. nov. |
Diutius pallidus Opitz , new species
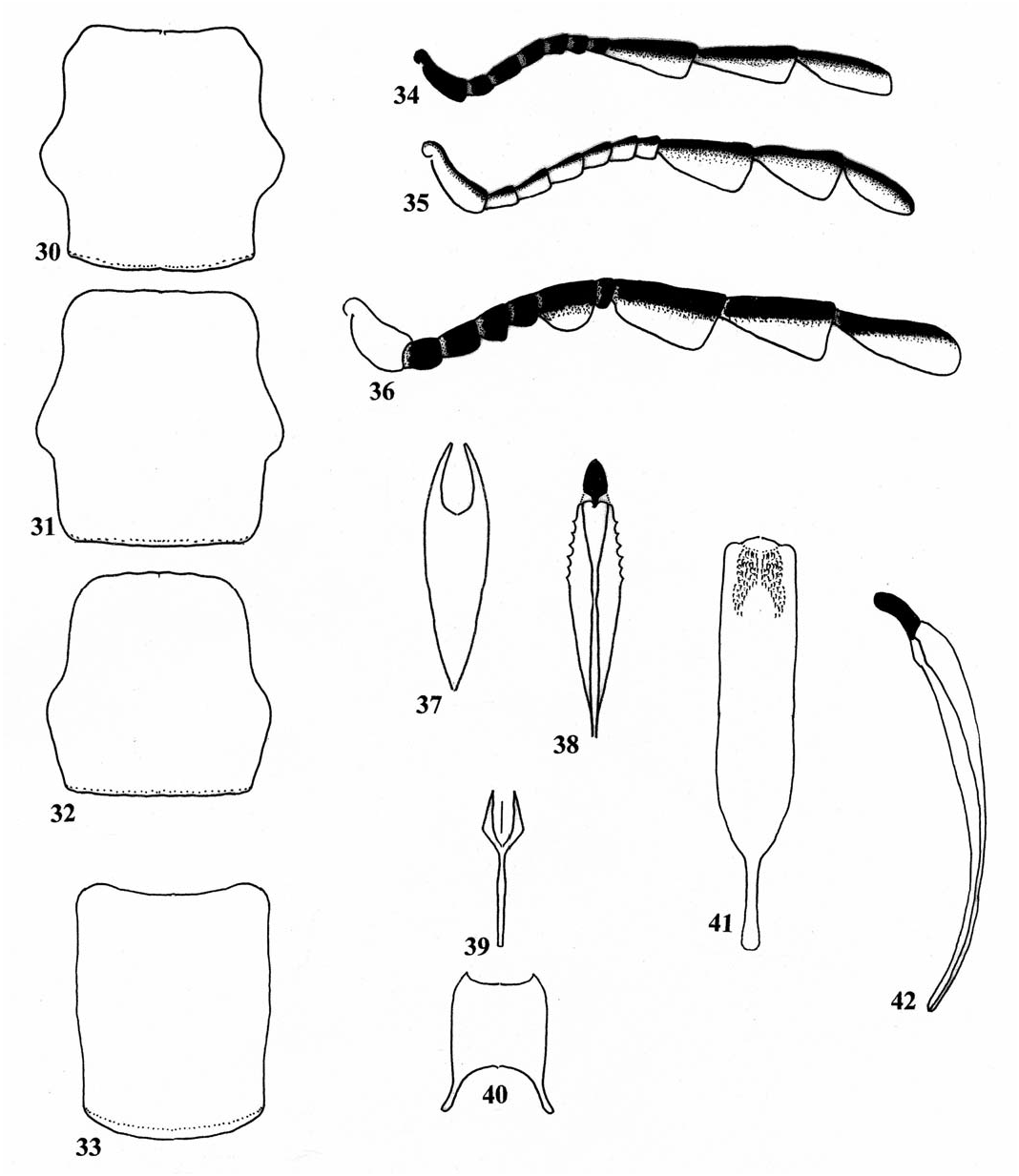
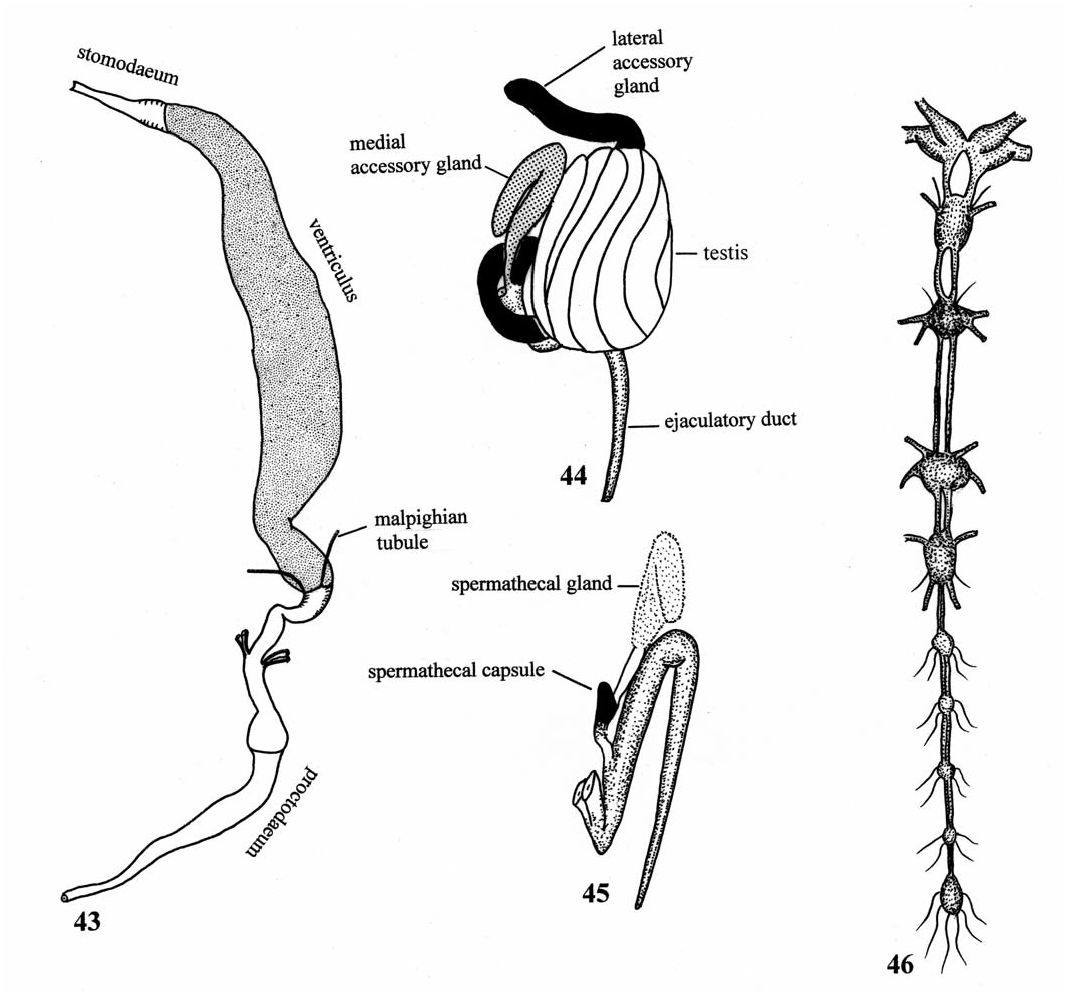
( Figs. 32, 34, 37, 38 View Figs , 43–46 View Figs , 59 View Fig , 62 View Figs )
Holotype. ♂. BOLIVIA: Santa Cruz, 3.7 km SSE Buena Vista , Hotel Flora & Fauna, 430 km, 14-19-X-2000, coll. M.C. Thomas, tropical transition forest ( MNKM).
Paratypes. Six specimens. One from the same locality as the holotype ( FSCA) . BOLIVIA: Santa Cruz: Buena Vista , 18-25-X-1992, E. Giesbert ( FSCA, 1; WOPC, 1); idem, Flora @ Fauna Hotel, 22-26-X-2002, Morris & Wappes ( JEWC, 1); Potrerillo, del Guenda, 6-8 XII- 2011, 400 m, Morris & Wappes ( RFMC, 1): Cochabamba: Villa Tunari, 15-I-1981, malaise trap, R.C. Wilkerson ( WOPC, 1) .
Diagnosis. Only in the members of this species is the elytral disc uniformly light brown.
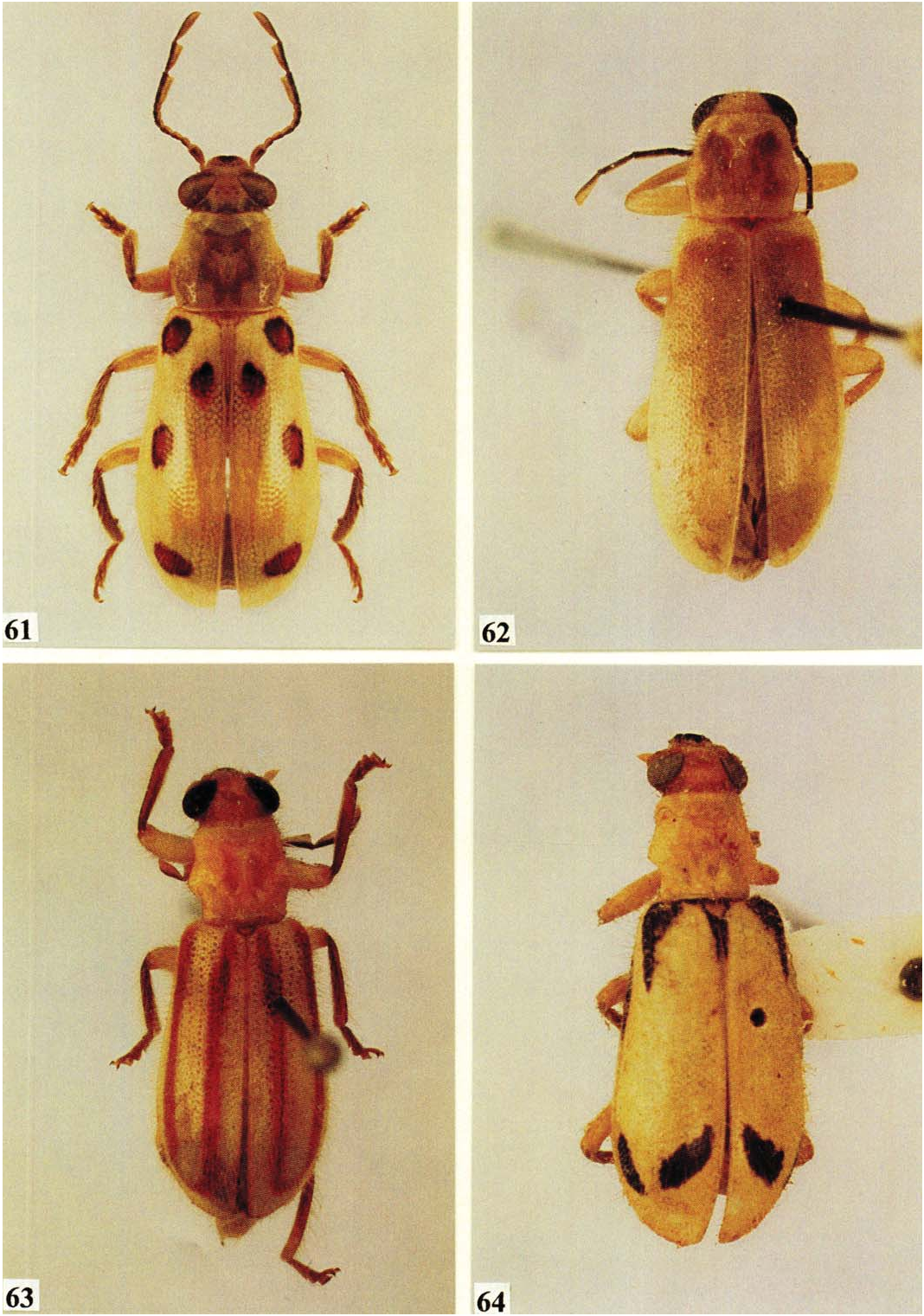
Description. Size: Length 12.0 mm; width 4.0 mm. Form: As in Fig. 62 View Figs . Integument: Cranium and pronotum yellow, pronotal disc slightly dark brown; elytra light brown, legs, pterothorax, and abdomen yellow. Head: Eyes wider than frons (30:20); capitular antennomeres very slen- der ( Fig. 34 View Figs ). Thorax: Pronotum quadrate (80:80), lateral tubercle faintly developed ( Fig. 32 View Figs ); epipleural fold gradually narrowed to elytral apex, asetiferous punctures absent; 1° setae absent. Abdomen: Male pygidium notched; aedeagus as in Figs. 37 and 38 View Figs .
Variation. Size: Length 9.0–12.0 mm; width 2.2–4.0 mm.
Natural History. Specimens were collected during October and January, two in a Malaise trap at 250 m elevation.
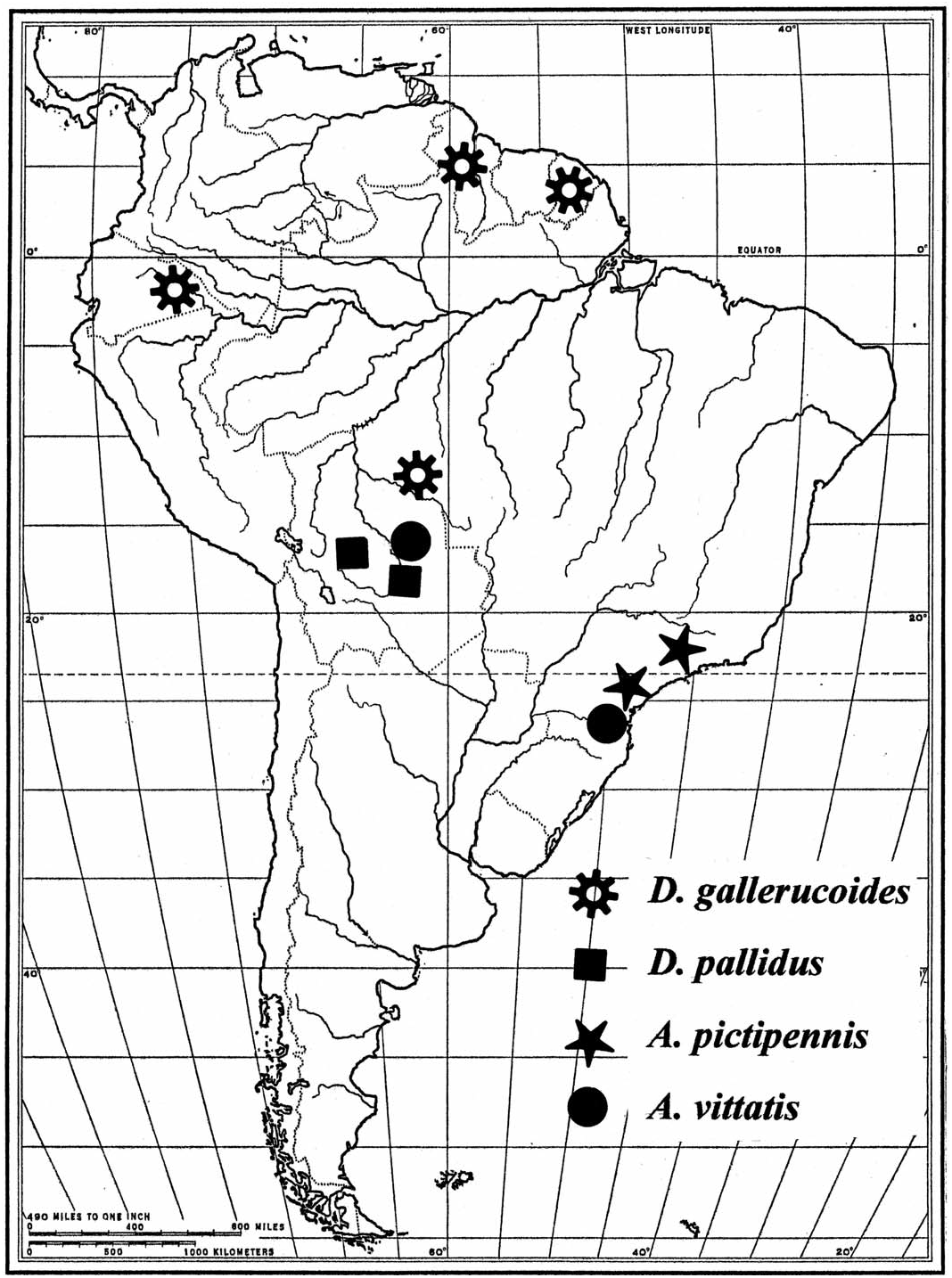
Distribution. Known only from Bolivia ( Fig. 59 View Fig ).
Etymology. The trivial name pallidus (= pale) is a Latin adjective. I refer to the pale condition of the elytral disc.
EVOLUTIONARY CONSIDERATIONS
A discussion of evolutionary relationships is an important component of taxonomic endeavors. Such discussions, if based on a wide variety of derived character states ( Hennig, 1966), have a good possibility of producing classifications that are informative and of heuristic value. This treatise involves four genera that are either monotypic or bitypic. It remains to be seen whether the species paucity in these taxa represents considerable rates of extinction, a manifestation of unavailability of specimens collected, or a result of geographic bias in collecting activities. Whatever the case, I have detailed a number of characteristics that warrant some discussion of kinships. It is understood that the following remarks are tentative and represent a beginning which may be augmented as more taxa and characteristics are discovered.
I suggest that the distribution of the taxa under consideration reflect historical vicariance, with the possible exception of A. impressocollis , which dispersed from ancestral South American clerofaunas ( Opitz 2005) to enter the more eastern Panamanian terrain, probably after the early closure of the Panamanian portal about 25 million years ago ( Ford 2006). The distributions of the other species roughly coincide with Quaternary forest refugia postulated by Haffer (1969) and Vanzolini and Williams (1970). The known distributions of these species coincide well with the South American clerofaunas described by Opitz (2005).
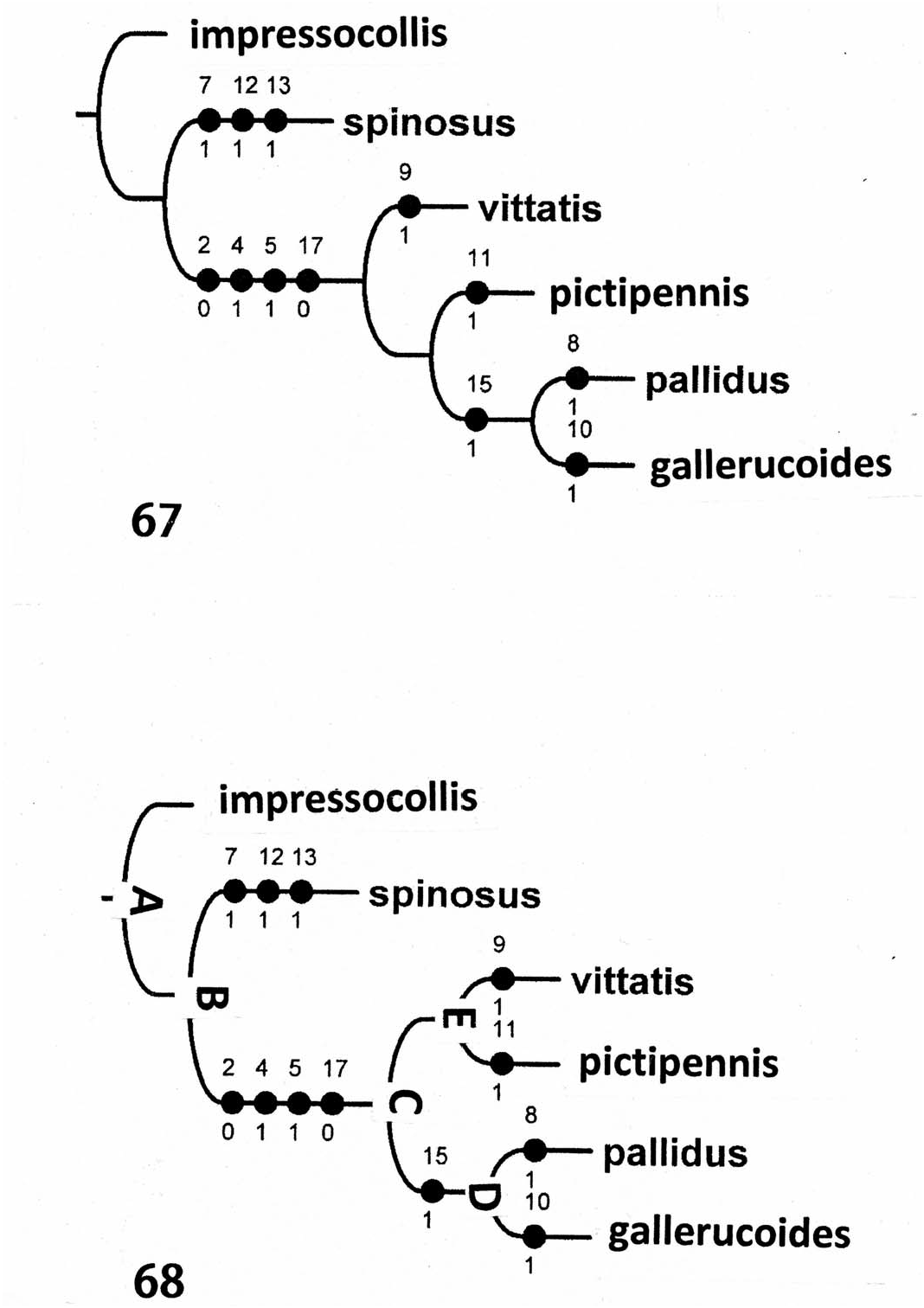
Analysis of the character matrix ( Table 1) produced two phylogenetic trees ( Figs. 67, 68 View Figs ). Both trees involved 15 steps, index of consistency of 100, and index of retention of 100. Heuristic analysis included maximum trees (hold) = 100, number of replications 9 (must), and multiple TBR = TBR (mult max) = 100. I have chosen to discuss tree number two ( Fig. 68 View Figs ) because it presents a more realistic relationship between D. pallidus and D. gallerucoides ; both species share the derived condition of the tarsal spur formula, which is 0-2-2.
Distributional evidence suggests that the ancestor of the taxa in question (ancestor A) originated in South America. This ancestor had a basic plan that included an elongated capitulum and an epipleuron that shifted from a lateral position to a more ventral position. Ancestor A generated the widely distributed Antennosus , which developed a bifid spicular fork and two wide indentations on the pronotum. The complementary stock generated ancestor B in which the phallobasic rod disappeared and the phallobasic struts became abbreviated. This progenitor generated Crusbatus whose species evolved an elongated pronotum, expanded funicular antennomeres, concavely margined pygidium, and spinous anterior margin of the protibia. Ancestor B also produced a line of evolution leading to ancestor C characterized by having a flat pronotum. Then, in one line of the progenitors of ancestor C (which led to progenitor D) the protibiae lost their tibial spur and evolved a 0-2-2 tibial spur formula. Progenitor D evolved D. pallidus characterized by an infuscated pronotum and D. gallerucoides characterized by brown maculae on the elytral disc. Ancestor C also generated progenitor E which evolved A. vittatis characterized by vittate elytra and A. pictipennis whose derived attributes include a dark humeral margin.
| FSCA |
Florida State Collection of Arthropods, The Museum of Entomology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |