Wolcott (1948:5)
|
publication ID |
https://doi.org/10.1649/587 |
|
persistent identifier |
https://treatment.plazi.org/id/03F5CB5E-FFEC-FFE5-724F-FC2A24F2FDCF |
|
treatment provided by |
Tatiana |
|
scientific name |
Wolcott (1948:5) |
| status |
|
Redescription of Macrosiagon mutilata ( Gerstaecker, 1855)
Figs. 2 View Figs , 4–5 View Figs , 8 View Figs , 10 View Figs
Rhipiphorus mutilatus Gerstaecker 1855:32 . Emenadia mutilatus in Lacordaire 1859:628. Emenadia mutilata in Gemminger and von Harold 1870:2121. Rhipiphorus mutillatus [ sic] in Quedenfelt 1886:128. As synonym of Emenadia discicollis (Gerstaecker) in Champion 1891:358. As variety of Macrosiagon discicolle (Gerstaecker) in Csiki 1913:12. As synonym of Macrosiagon discicollis (Gerstaecker) in Rivnay 1929:39. As variety of Macrosiagon discicolle (Gerstaecker) in Blackwelder 1944:480. Hereby removed from junior subjective synonymy with Macrosiagon vittata (Erichson) (¼ Macrosiagon discicollis (Gerstaecker) , see below).
Rhipiphorus quadrimaculatus Gerstaecker 1855:33 (not Gyllenhal 1817:36). Emenadia quadrimaculatus in Lacordaire 1859:628. Emenadia quadrimaculata in Gemminger and von Harold 1870:2122. Rhipiphorus quadrimaculatus in Quedenfelt 1886:128. As synonym of Emenadia discicollis (Gerstaecker) in Champion 1891:358. As variety of Macrosiagon discicollis (Gerstaecker) in Csiki 1913:12. As synonym of Macrosiagon discicollis (Gerstaecker) in Rivnay 1929:39. As variety of Macrosiagon discicollis (Gerstaecker) in Blackwelder 1944:480. Macrosiagon quadrimaculata (Gerstaecker) hereby removed from junior subjective synonymy with Macrosiagon vittata (Erichson) (¼ Macrosiagon discicollis (Gerstaecker) , see below) and placed in junior subjective synonymy with Macrosiagon mutilata (Gerstaecker) , new synonymy.
Emenadia melanoptera Chevrolat 1877 :ix. As synonym of Emenadia discicollis (Gerstaecker) in Champion 1891:358. As variety of Macrosiagon discicollis (Gerstaecker) in Csiki 1913:12. As synonym of Macrosiagon discicollis (Gerstaecker) in Rivnay 1929:39. As variety of Macrosiagon discicollis (Gerstaecker) in Blackwelder 1944:480. Hereby removed from junior subjective synonymy with Macrosiagon vittata (Erichson) (¼ Macrosiagon discicollis (Gerstaecker) , see below) and placed in junior subjective synonymy with Macrosiagon mutilata (Gerstaecker) , new synonymy.
Emenadia vitraci Fleutiaux and Sallé 1889:432 . As synonym of Emenadia discicollis (Gerstaecker) in Champion 1891:358. As synonym of Macrosiagon discicollis (Gerstaecker) in Csiki 1913:12. As synonym of Macrosiagon discicollis (Gerstaecker) in Rivnay 1929:39. As synonym of variety Macrosiagon quadrimaculatum (Gerstaecker) , a synonym of Macrosiagon discicolle (Gerstaecker) in Blackwelder 1944:480. Hereby removed from junior subjective synonymy with Macrosiagon vittata (Erichson) (¼ Macrosiagon discicollis (Gerstaecker) , see below) and placed in junior subjective synonymy with Macrosiagon mutilata (Gerstaecker) , new synonymy.
Description, Male. General body form typical of Macrosiagon , although smaller, vertex much less elevated, dorso-ventral length of head shorter, and elytra less acuminate than typical ( Fig. 2 View Figs ). Overall length from frons to elytral apices 3.5–5.2 mm, (avg. 4.4 mm, n ¼ 37). Color extremely variable; in all cases in which sex has been determined males with head entirely brown, pronotum ranging from entirely brown with yellow-orange posterior angles in most specimens to only anterior portion of disc brown in a few specimens. Elytra entirely brown in most specimens, yellow-orange with varying degrees of brown at the humeral and apical aspects in some. Color of thorax and legs variable, generally brown with varying degrees of yellow-orange; legs brown and abdomen yellow with brownish pygidium in most specimens. Wings fuscous.
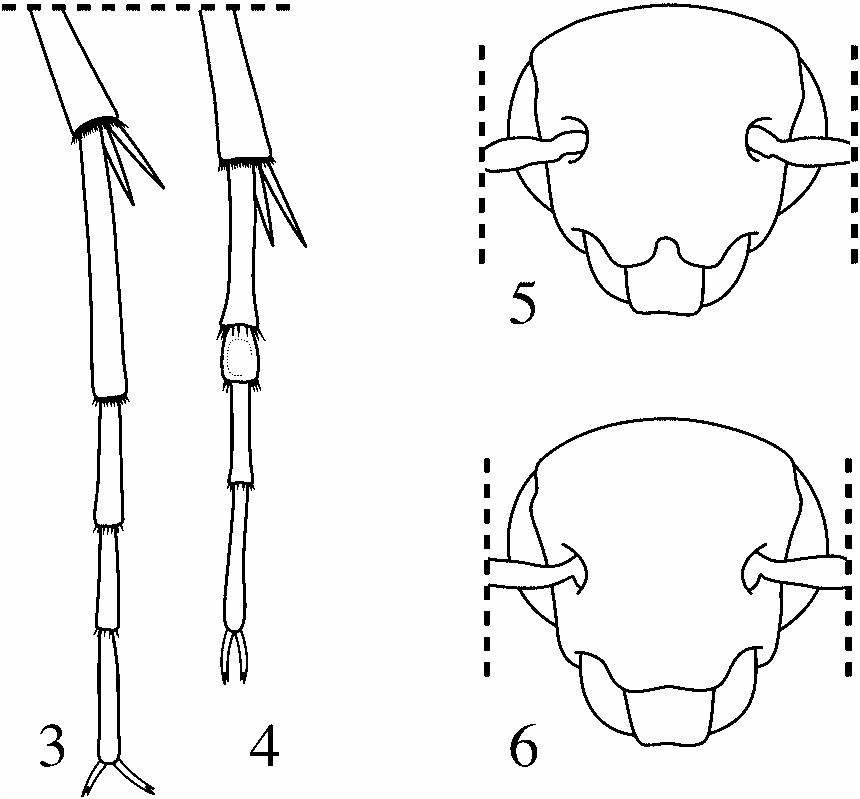
Head small, somewhat hemispherical, compressed antero-posteriorly, more rounded than typical for species. Vertex transversely planar, gently convex antero-posteriorly to antennal bases, with scattered, very weak punctures, appearing nearly glabrous. Area of frons from base of antennae to frontoclypeal margin less convex, appearing more or less flattened in profile ( Fig. 2 View Figs ), weak punctures gradually becoming more dense towards frontoclypeal margin and between lower margin of eye and base of mandible. Frontoclypeal margin with distinctive medial notch ( Fig. 5). Labrum visible beneath frontoclypeus in most specimens, densely and relatively strongly punctate, broadly and shallowly emarginate at apex with a fringe of long, downward facing setae.
Antennae typical of males of the genus, essentially similar to that of M. gracilis except antennomere I slightly more subconical than subcylindrical.
Pronotum essentially similar to that of M. gracilis in overall shape except posterior angles not as developed, not extending to the posterior margin of the mesepimeron in most specimens and ventral margin of the lateral aspect of pronotum uniformly arched throughout. Posterior lobe of pronotum a slightly convex, bluntly obtuse triangular flange covering the scutellum in nearly all specimens, revealing the posterior apex of scutellum in a few. Pronotal disc regularly convex, smooth and shining; its surface with relatively scattered punctures with recumbent setae. Prosternum a flattened, acutely pointed triangular projection extending nearly the entire length of the front coxae, separating them.
Lateral and ventral aspects of thorax more or less typical of genus, more strongly and densely punctured than pronotum. Lateral aspect of meso- and metathorax slightly convex, though not visible from above as in some species of the genus. Mesosternum similar in form to prosternum except more broad, not as acute and lying more ventral to mesocoxae, not effectively separating them. Metasternum broad, expanded, typical of genus.
Legs typical, densely punctate and setose; ventral surface of apical 1/4 of profemora excavated with row of dense setae; apices of all tibiae with two ventral spurs. Tarsi 5-5-4, all tarsomeres more or less cylindrical except second metatarsomere. Second metatarsomere subcylindrical except dorsal surface flattened, slightly excavated and glabrous ( Fig. 4 View Figs ); ratio of length of tarsomere II to length of III approximately 2:3.5. Claws typical; long, curved, laterally compressed and finely bifid at apex.
Elytra subparallel along lateral margins, broadest at middle, tapering slightly towards apex, strongly divergent medially only in apical 1/3; outer apical angle nearly 90 8, arching uniformly towards medial margin, giving an unusually blunt appearance to the elytral apices. Elytra more or less uniformly convex, punctation and setation similar to thorax.
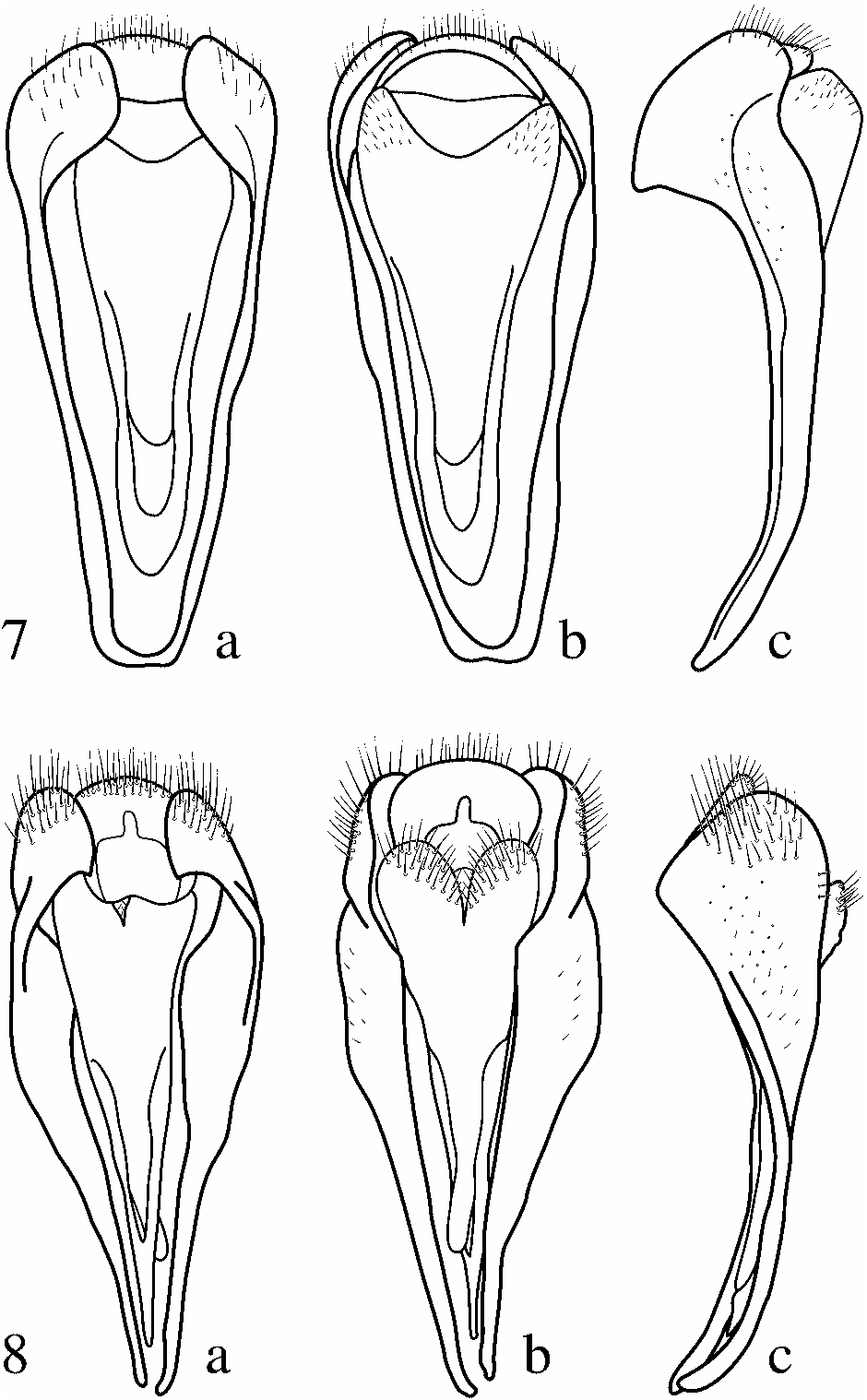
Abdomen typical of genus, punctation and setation similar to thorax. Abdominal segment IX ( Fig. 8a–c View Figs ) trilobed dorsally, median lobe overlapped dorsally by lateral lobes, median lobe less well sclerotized than lateral lobes with the anterior portion membranous, appearing crescent-like from dorsal view; lateral lobes nearly uniformly convex dorso-ventrally but planar anteroposteriorly, extending antero-ventrally, becoming nearly parallel at the apices, apices not quite touching and bent slightly dorsally; the three dorsal lobes with scattered setae. Posterior aspect of ventral lobe of segment IX weakly sclerotized, deeply bi-lobed, apical half of lobes with setae. Anterior aspect of ventral lobe of segment IX more strongly sclerotized, extending anteriorly as spine located between anterior processes of lateral lobes.
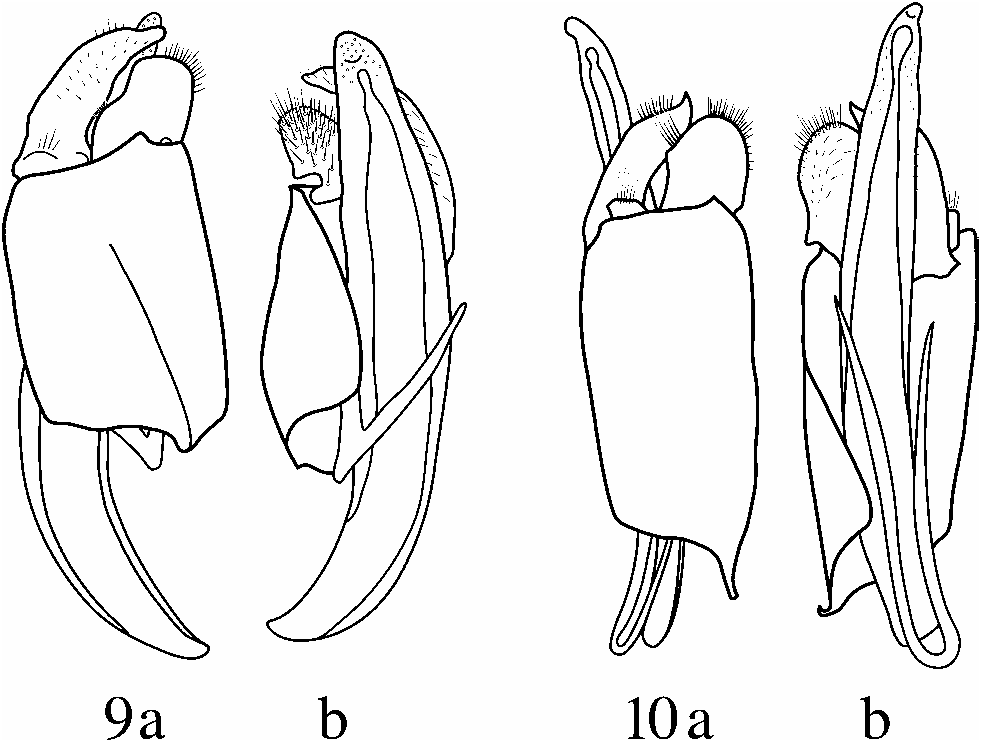
Gonoforceps ( Fig. 10a, b View Figs ) large, asymmetrical, tightly articulated to dorsal aspect of body of tegmen; one forcep resembling a laterally-projecting thick, curved tooth or hook with a separate, small, dorsally located flange, the other a dorso-ventrally aligned irregular, broad flange. Ventral aspects of hook and dorso-ventrally aligned flange lined with setae, smaller dorsal flange with a few elongate setae. Tegmen resembles a curved plate surrounding median lobe, asymmetrical, anterior aspect curving ventrally and to the side. Ventral baculi poorly sclerotized, inconspicuous, fused into narrow, parallel-sided stirrup-shaped sclerite attatched membranously to ventral aspect of tegmen. Median lobe relatively broad, gently dorsally arched, approximately 1.5 3 length of tegmen, with blunt, ventral tooth at apex.
Female. In all cases in which sex has been determined, females have pronotum, thorax and abdomen yellow-orange with brownish pygidium. Head yellow-orange in most specimens, more brownish in a few. Elytra variable; basal and apical 1/3 black, middle 1/3 yellow-orange or elytra entirely black in most specimens, elytra nearly all yellow-orange in one specimen.
Female external morphology identical to male except for antennae. Antennomeres I and II as in male; antennomeres III–X subequal, cylindrical, uniramous. Rami somewhat variable; more or less cylindrical, tapered to a dull point at apex and progressively more compressed antero-posteriorly through the penultimate antennomere. Rami produced at a consistent angle from antennomere in most specimens, becoming more erect towards apex in a few. In some specimens, rami are more tapered and less compressed. In most specimens, rami increase in length until antennomere VII, then decrease in length in subsequent antennomeres, though in some specimens rami appear nearly uniform in length throughout. Antennomere XI suboval, compressed laterally. Antennomeres and rami with few, scattered suberect sensillae.
Immature Stages. Unknown.
Taxonomic Notes. Gerstaecker (1855) described both M. mutilata and M. quadrimaculata in the same work, giving neither of these subjectively synonymous names objective precedence. However, the specific epithet quadrimaculatum was first proposed by Gyllenhal (1817) for a different, congeneric species that has since been subjectively synonymized under M. tricuspidata (Lepechin) . Therefore, as M. quadrimaculata (Gerstaecker) is a junior primary homonym, I invoke Art. 24.2 (Determination by the First Reviser) and its recommendation 24A ( ICZN 1999) in selecting M. mutilata as the valid, senior subjective synonym. Unfortunately, there is some confusion over the type locality of M. mutilata ; the labels associated with the lecto- and paralectotypes (see below) read ‘‘St. Jean’’ as the collection locality although Gerstaecker lists the country of origin in the original description as Colombia. This discrepancy is noteworthy in that the types agree in every aspect with all other specimens collected in the Caribbean (excluding Trinidad); they exhibit a frontoclypeal notch and the distinctive female color pattern not exhibited by those specimens collected on mainland Central and South America. While it is conceivable that there exists a mainland population of the Caribbean species, it is almost certain that Gerstaecker was in error in attributing Colombia as the country of origin of these specimens. In his work on the insects of Puerto Rico, Wolcott (1948:5) paraphrases from a work written by the collector of the type specimens, C. Moritz, stating that ‘‘... coming from the Danish islands of St. Thomas and St. John, [Mortiz] landed at Arecibo [ Puerto Rico] on 17 February 1835...’’ The island of St. John (spelled ‘‘St. Jean’’ in the original ( Moritz 1836)) in what is now the U.S. Virgin Islands, then, is the most likely country of origin of the type specimens of M. mutilata .
Champion (1891) considered M. quadrimaculata , M. melanoptera (Chevrolat) , M. vitraci (Fleutiaux and Salle´), and M. mutilata (Gerstaecker) to be color variants of M. discicollis (Gerstaecker) (which is now synonymized under M. vittata (Erichson)) . Csiki (1913), Rivnay (1929), and Blackwelder (1944) accepted this arrangement in their respective treatments of this species. However, using the form of the frontoclypeal margin as a guide, it is clear that M. mutilata is indeed a valid species and that M. melanoptera and M. vitraci should be placed as junior synonyms under M. mutilata and not M. vittata .
Type Material. Gerstaecker (1855) described Rhipiphorus mutilatus from two females collected from ‘‘Columbiam’’ (see above). The first specimen is labelled as follows: ‘‘29243’’ typeface /‘‘St. Jean[,] Mor. [coll.]’’ handwriting on bluish-green paper /‘‘ mutilatus Gerst. *’’ handwriting on bluish-green paper /‘‘TYPE’’ typeface on orange paper /‘‘ mutilatus Gerst. ’’ handwriting /‘‘Zool. Mus. Berlin’’ typeface. The second specimen is labelled as follows: ‘‘St. Jean[,] Moritz [coll.] Nr. 29243’’ handwriting on bluish-green paper /‘‘29243’’ handwriting /‘‘PARATYPUS’’ typeface on orange paper /‘‘Zool. Mus. Berlin’’ typeface. As neither of these specimens qualify as a holotype according to article 73.1 of the International Code of Zoological Nomenclature (1999), I hereby designate the first specimen to be the lectotype and the second to be a paralectotype of M. mutilata and have affixed typefaced labels to the respective specimens to this effect. The lectotype is completely intact and the paralectotype is intact except for lacking the last three tarsomeres and claw on the right fore-leg. Both specimens are deposited at the ZMHB.
Gerstaecker (1855) described Rhipiphorus quadrimaculatus from one male and one female collected in Cuba. The male is labelled as follows: ‘‘29244’’ typeface /‘‘ Cuba [,] Otto [coll.]’’ handwriting on bluish-green paper /‘‘4maculatus Gerst.*’’ handwriting on bluish-green paper /‘‘TYPE’’ typeface on orange paper /‘‘4maculatu [sic] Gerst.’’ handwriting /‘‘Zool. Mus. Berlin’’ typeface. The female is labelled as follows: ‘‘29244’’ handwriting /‘‘ Cuba [,] Otto [coll.] Nr. 29244’’ handwriting on bluish-green paper / ‘‘PARATYPUS’’ typeface on orange paper /‘‘Zool. Mus. Berlin’’ typeface. Again , as neither of these specimens qualify as a holotype, I hereby designate the male specimen to be the lectotype and the female to be a paralectotype of M. quadrimaculata (Gerstaecker) and have affixed typefaced labels to the respective specimens to this effect. The lectotype is intact except for lacking antennomeres III–XI from the right antenna and tarsomeres III–VI on the left protarsus. The paralectotype appears to be completely intact. Both specimens are deposited in the ZMHB .
Chevrolat (1877) described M. melanoptera under the then accepted generic name Emenadia from an undetermined number of specimens collected in Puerto Rico. The type specimen(s) were not found after a brief search at the MNHN and so were not examined for this work.
Similarly, Fleutiaux and Sallé (1889) described M. vitraci under the generic name Emenadia from an undetermined number of specimens collected in Guadeloupe, W.I. The type specimen(s) of this species were also not found at the MNHN. However, one specimen at the BMNH identified as M. vitraci by Champion (1891) was located by the author and is clearly another example of M. mutilata .
Other Material Examined. 37 specimens total. CUBA : 1 male, Baragua´ , 4-V- 1930, ‘‘grasses,’’ L.D. Christensen coll., ( MCZC) ; 1 female, Soledad, Cienfeugos , Jan– Feb 1927, C.T. and B.B. Brues [colls.] ( MCZC) ; 1 specimen (sex undetermined), ‘‘ Cuba’ ’ ( MNHC) ; 1 female, Los Alpes , C. Zapata Mtz, III-1989, J.A. Genaro, coll., ‘‘en romerillo’’ ( MNHC) ; 1 female, Camoa [sic?], Habana [Prov.], Cuba , 16-VII-1950 ( MNHC) ; 1 female, Matanzas, Matanzas Prov., V-1933, Colección de M. Barro ( MNHC) ; 1 specimen (sex undetermined), Gabaiguan Sta [tion] Glara , 27-II-1931, S.G. Brunner [coll.] ( MNHC) ; 1 male, Baracoa, Habana [Prov.], Cuba , VIII-1966, P. Alayo, coll. ( MNHC) ; 1 male, Havana, Baker [coll.] ( USNM) ; 1 female, ‘‘ Cuba, W.I. ’’, M.S. Roig [coll.] ( USNM) . DOMINICAN REPUBLIC: 1 male, Sanchez , 7 to 12-VI-1915 ( AMNH) ; 1 male, St. Domingo , Sanchez, W.I. [?], P.G. Russell coll. ( USNM) . GUADELOUPE: 1 male, Domaine Duclos , 7-VII-1960, P. and C. Vaurie [colls.] ( AMNH) ; 1 male, Guadal. [oupe?] ( BMNH) . JAMAICA: 1 female, Georges Valley , Manchester, 18-XI-1919, about 2,000 ft, ( AMNH) ; 1 male, St. Ann Parish, Claremont , 12-VIII-1985, G.C. Eickwort [coll.] ( CUIC) ; 1 female Trelawny Parish, Windsor , 10- VIII-1985, G.C. Eickwort [coll.] ( CUIC) ; 1 female, 4 mi south Christiana , VII-1961, J. Maldonado C. [coll.] ( USNM) . MEXICO?: 1 female, ‘‘ Mex’ ’, B.C.A. Col. IV.2. ( BMNH) . PUERTO RICO: 2 males, 1 specimen (sex undetermined) Mayagüez, Fed. Exp. Sta. , 19-VII-1914, R.H. Van Zwalenburg coll. ( AMNH) ; 1 male, same, 12-VIII- 1916 ( AMNH) ; 2 females, same, 12-X-1916 ( AMNH) ; 1 male, Mayagüez, Fed. Exp. Sta. , 2-X-1914 ( AMNH) ; 1 male, same, 29-IX-1916 ( AMNH) ; 1 specimen (sex undetermined), same, 5-IX-1914, ‘‘ on Erigeron sp. ,’’ ( AMNH) ; 1 female, Anasco, Fed. Exp. Sta. , 14-IX-1917 ( AMNH) ; 1 male, Mayagüez, I-1929, Coll. S.T. Danforth ( MCZC) ; 1 male, 1 female, ‘‘ Porto Rico’ ’ ( MNHN) ; 1 female, L. Tortuguero , 11-VII- 1944 ( USNM) ; 1 male, Bayamon, 26-XII-1932, ‘‘on Jamaican sorrel pod’’ ( USNM) ; 1 female, Bayamon, 20-III-1934, Fayon, Mills [and] Anderson [colls.] ( USNM) ; 1 male, Trujillo Alto, 12-XII-1933, ‘‘on palm leaf,’’ Anderson and Mills [colls.] ( USNM) ; 1 female, Rio Piedras , 2-V-1912, T.H. Jones coll. ( USNM) . LOCALITY UNKNOWN: 1 male, ‘‘71’’ ( ZMHB) .
Distribution. Except for one suspect record of M. mutilata being taken in Mexico (most likely either a labeling error or else the result of a unique introduction event) and the confusion over the type locality, this species is confined to the islands of the Greater Antilles and the northern reaches of the Lesser Antilles. Although one of its probable host species, Exomalopsis similis Cresson , has been taken in Florida ( Mitchell 1962; see below) and there are a number of other Macrosiagon species that have been recorded from both the Caribbean region and the continental U.S. ( e.g., M. cruenta (Germar) , M. octomaculata , and M. limbata (Fabricius)) , M. mutilata has not been taken in the United States. Macrosiagon vittata , the sister species of M. mutilata , is restricted to mainland Central and South America except for three specimens taken on the island of Trinidad. These specimens are undoubtedly M. vittata and show no evidence of introgression with M. mutilata .
Bionomics. No information regarding the biology of M. mutilata appears in the literature other than ‘‘On the tips of grasses and particularly from vetiver ([ Poaceae :] Andropogon muricatum )’’ as noted in the description of M. vitraci (Fleutiaux and Salle´). Raw (1977) states that several Macrosiagon specimens were taken from Exomalopsis globosa (Fabricius) ( Apidae : Apinae) and E. similis nests in Jamaica. Because no other Macrosiagon species are known from Jamaica and M. vittata is known to parasitize Exomalopsis bruesi Cockerell (Rosen 1997) , it is likely that these specimens were in fact M. mutilata , although attempts to examine the voucher specimens were unsuccessful. The biological information derived from the collection data on the labels associated with the above specimens is scant and likely insignificant.
Diagnostic Characters. Macrosiagon mutilata is distinguished from all other Macrosiagon species except M. vittata by the combination of its 2-2-2 tibial spur formula and the fact that the second metatarsomere is flattened dorsally and shorter than the third. Although M. mutilata and M. vittata are nearly identical, they can be easily distinguished by the fact that the frontoclypeal margin of M. mutilata is unmistakably deeply notched whereas the frontoclypeal margin of M. vittata is either straight or only slightly emarginate. Other than the unique and possibly erroneous collection of a single M. mutilata specimen in Mexico, it appears that the geographic ranges of these two species are completely disjunct and that collection locality is a good, though not necessarily foolproof, indication of species identity.
Comments. Although relatively uniform in size, M. mutilata varies considerably in coloration within and between the sexes, a fact that has undoubtedly contributed to its description under several binomials. Vaurie (1955) was the first to notice the fairly consistent differences in coloration between the sexes. While examination of the above specimens confirmed this phenomenon, no correlation between coloration and geography could be detected. There is some slight variation in the shape of the pronotal lobe in this species; in some specimens it appears as a blunt triangular flange whereas in other it is more rounded overall, appearing nearly semi-circular rather than triangular. The only other significant variation is in the form of the antennal flabella of the females as described above. These variations do not appear to be consistent in any way and are not deemed worthy of constituting specific distinctions.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
Wolcott (1948:5)
| Falin, Zachary H. 2004 |
Rhipiphorus mutilatus
| Blackwelder 1944: 480 |
| Rivnay 1929: 39 |
| Csiki 1913: 12 |
| Champion 1891: 358 |
| Quedenfelt 1886: 128 |
| Lacordaire 1859: 628 |
| Gerstaecker 1855: 32 |
Rhipiphorus quadrimaculatus
| Blackwelder 1944: 480 |
| Rivnay 1929: 39 |
| Csiki 1913: 12 |
| Champion 1891: 358 |
| Quedenfelt 1886: 128 |
| Lacordaire 1859: 628 |
| Gerstaecker 1855: 33 |
| Gyllenhal 1817: 36 |