Bolitoglossa aurae, Kubicki, Brian & Arias, Erick, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4184.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:26A16620-CAAE-4B1C-AB63-61CB2611C5BF |
|
DOI |
https://doi.org/10.5281/zenodo.6063808 |
|
persistent identifier |
https://treatment.plazi.org/id/03F687D7-FFAD-C24B-F5D8-F57DFDB9CB0C |
|
treatment provided by |
Plazi |
|
scientific name |
Bolitoglossa aurae |
| status |
sp. nov. |
Bolitoglossa aurae View in CoL sp. nov.
Aura’s golden salamander ( Figures 3–9 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 )
Holotype. UCR 22842, an adult female from Costa Rica: Provincia de Limón: Cantón de Turrialba: Distrito de Chirripó: in the vicinity of Moravia de Chirripó, ca . 1300 m a.s.l., collected by Brian Kubicki, in the company of Maximo Flores and Aura Reyes on 22 March 2013.
Generic Placement. Assigned to the genus Bolitoglossa due to having fewer than 14 costal grooves and lacking a sublingual fold, and to the subgenus Eladinea based the molecular evidence presented herein.
Diagnosis. The combination of the following characteristics can be used to distinguish Bolitoglossa aurae from other described species in the genus: (1) unique coloration consisting of a light yellow ground color of the body, limbs, and tail, and having a dark brown middorsal stripe on the head and body and a pair of thin dark brown lateral stripes running from behind the eye posteriorly onto the tail ( Fig. 3 View FIGURE 3 ); (2) iridescent green chromatophores scattered throughout the dorsal surfaces of the head, body, and tail, at times resulting in an evident greenish hue; (3) relatively long prehensile tail, 57.9 % of total length, or 137 % of SL; (4) few premaxillary teeth, [3 total] in relation to SL; (5) numerous vomerine teeth [32] and maxillary teeth [60] in relation to SL; (6) relatively short truncate digits with moderate to extensive webbing on the hands and feet.
Comparisons. Bolitoglossa aurae is differentiated from members of the subgenus Eladinea by its 16S and cyt b mtDNA distances. Since B. aurae is only known to occur in Costa Rica and molecular evidence strongly supports it forming part of the Bolitoglossa robinsoni clade within the subgenus Eladinea, phenotypic comparisons are presented here only with respect to members of that clade (i.e., B. aureogularis , B. jugivagans , and B. robinsoni ), which are endemic to southeastern Costa Rica and northwestern Panama .
Contrasting characteristics for Bolitoglossa aurae are presented in parentheses. Bolitoglossa aureogularis Boza-Oviedo et al., 2012 can be distinguished from B. aurae by having a tan to reddish-brown dorsum with numerous dark brown streaks, black flanks and a pair of dirty white patches along the ventrolateral surface of the ventrum, and a dark midventral stripe (dorsum uniform light yellow with a thin dark brown middorsal stripe on the trunk, flanks light yellow to light brown [subject to metachrosis], ventrum uniform translucent yellow and lacking any evident or contrasting light or dark dermal pigmentation or midventral stripe); 6.5 costal folds between adpressed limbs [accuracy of costal folds count confirmed in Hertz et al. 2013] (4.5 costal folds between adpressed limbs); relatively short broadly rounded snout, SnL = 27.9 % of HeW (relatively longer and narrow truncate snout, SnL = 31.0 % of HeW); relatively fewer maxillary teeth, MT/SL = 1.2 (MT/SL = 1.5); more premaxillary teeth, 6 PMT (fewer premaxillary teeth, 3 PMT); and longer digits, LT3 = 32.6 % of FoW (shorter digits, LT3 = 24.4 % of FoW). Bolitoglossa jugivagans Hertz et al., 2013 has a heavy concentration of darker pigmentation on the dorsal surfaces of the head, body, tail, and limbs that is reddish-brown with lighter streaks (dorsal surfaces of the head, body, tail, and limbs having a uniform light yellow coloration lacking any contrasting darker dermal pigmentation with the exception of a thin broken dark brown middorsal stripe on the body, which forms two thin separating lines between the base of the neck and the posterior/inner margins of the orbits); tips of the digits on the hands and feet are rounded (tips of the digits on the hands and feet truncate); relatively shorter tail, TaL = 124 % of SL (TaL = 137 % of SL); narrower internarial distance, IND = 23.3 % of HeW (wider internarial distance, IND = 27.6 % of HeW); longer snout, SnL = 41.9 % of HeW (shorter snout, SnL = 31 % of HeW); relatively shorter front limbs, FLL = 15.4 % of SL (relatively longer limbs, FLL = 20.5 % of SL); narrower feet, FoW = 65.1 % of HeW (slightly wider feet, FoW = 70.7 % of HeW); longer digits, LT3 = 46.4 % of FoW (shorter digits, LT3 = 24.4 % of FoW); relatively smaller eyes, EW = 72.2 % of SnL (relatively larger eyes, EW = 116.7 % of SnL). Bolitoglossa robinsoni Bolaños and Wake, 2009 has a heavy concentration of dark pigmentation on the dorsal surfaces of the head, body, tail, and limbs (dorsal surfaces of the head, body, tail, and limbs having a uniform light yellow coloration lacking any contrasting darker dermal pigmentation, with the exception of a thin broken dark brown middorsal stripe on the body, which forms two thin separating lines between the base of the neck and the posterior/inner margin of the orbits); large robust species with a tail shorter than SL, TaL = 94 % of SL (slender species with a longer tail, TaL = 137 % of SL); relatively fewer maxillary and vomerine teeth, MT/SL = 1.0, VT/SL = 0.3 (MT/SL = 1.5, VT/SL = 0.8); shorter trunk, AGL = 53.5 % of SL, with 2 costal folds between adpressed limbs (relatively longer trunk, AGL = 58 % of SL, with 4.5 costal folds between adpressed limbs); wide head, HeW = 30 % of AGL (narrower head, HeW = 24.7 % of AGL); longer limbs, HLL = 26.1 % of SL and FLL = 25.4 % of SL (relatively shorter limbs, HLL = 21.5 % of SL and FLL = 20.5 % of SL); wider hands and longer digits on the feet, HaW = 58.8 % of HeW and LT3 = 36.5 % of FoW (relatively narrower hands and shorter digits, Haw = 51.7 % of HeW and LT3 = 24.4 % of FoW); and relatively smaller eyes, EW = 82.3% of SnL (relatively larger eyes, EW = 116.7% of SnL).
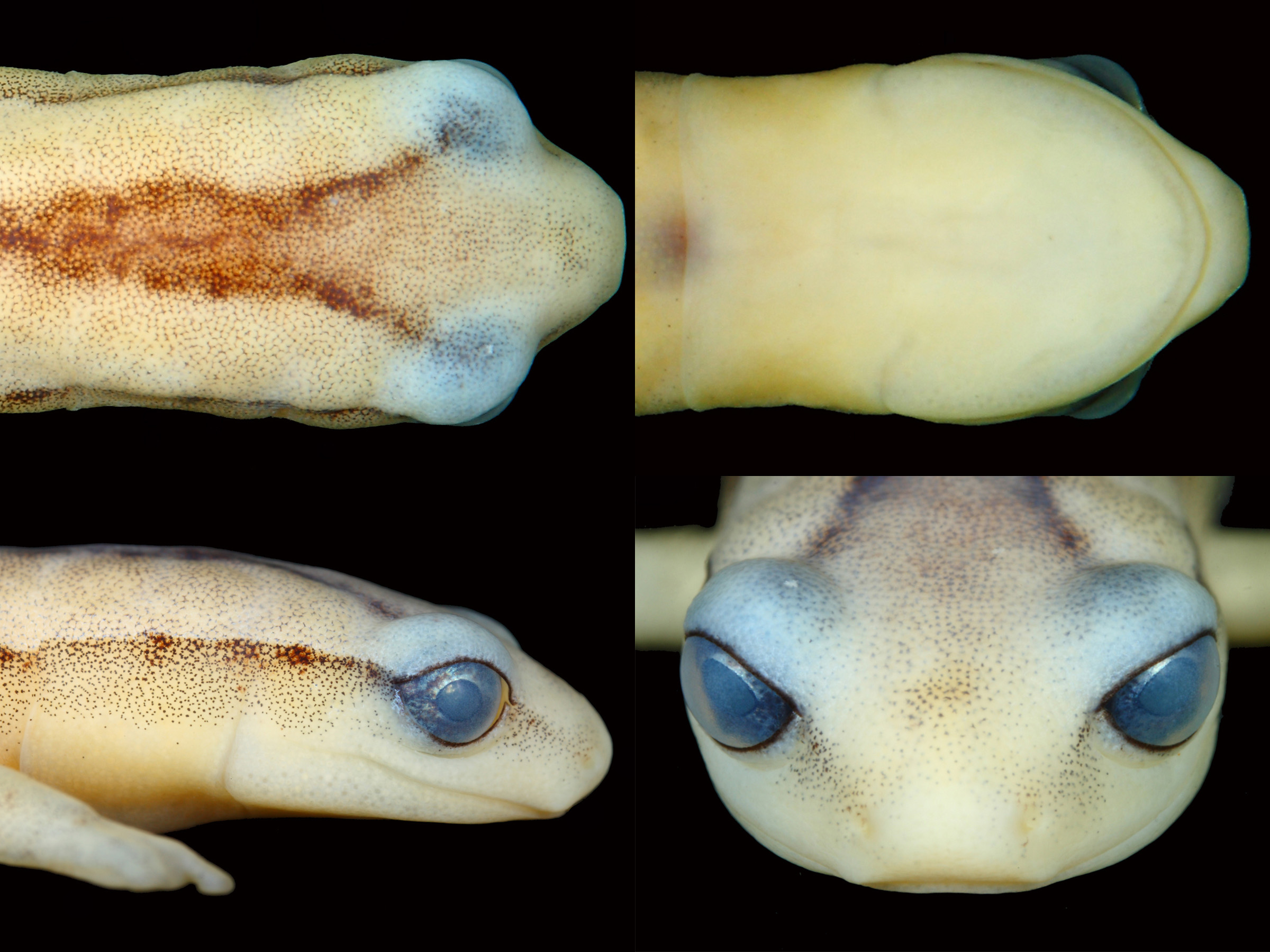
Description of holotype. Adult female having a SL of 40.5 mm. Head slightly wider than neck and shoulders (Hew 5.8 mm, NeW 5.2 mm, ShW 5.6 mm), with greatest width of head just posterior to the articulation of the jaws; snout raised anterodorsally, truncate in dorsal outline, but rounded in profile; snout relatively long (SnL 1.8 mm, 4.4 % of SL), with nearly terminal non-protruding tiny nostrils (LNH 0.09 mm, RNW 0.03 mm) directed laterally; internarial area slightly convex. Eyes relatively large (EW = 116 % of SnL), protruding beyond dorsal and ventral outline of head, directed anterolaterally, with a distinct suborbital groove. Top of head flat and smooth, lacking any contrasting interorbital dermal structure; canthus rostralis distinct and rounded; intercanthal area slightly convex, loreal region slightly concave. Poorly developed, but discernible rounded cirri (nasolabial protuberances) present on the tip of the snout, protruding slightly beyond the margin of the upper lip. Gular fold well-defined, starting on the lateral portion of the neck, below the dark lateral stripe, curving slightly posteriorly initially and continuing down onto the ventrum, crossing as an overall straight crease but with slight anterior curve in midventral region. In life a red heart was visible beneath the skin at the midventral gular fold, lying mostly posterior to gular fold. In ventral view, tip of snout protruding markedly beyond edge of lower lip. No mental gland visible under skin of anterior intermandibular region. Nuchal groove well defined, starting just posterior to jaw articulation, about level with lower margin of eye; groove has very slight posterior orientation on side of head, extending onto ventrum just medial to posterior edge of mandibular bones, terminating as an anteriorly oriented curve ( Fig. 4 View FIGURE 4 ).
Sublingual fold absent; tongue circular in shape, lacking distinct notches. Vomerine teeth numerous (14/18), present in two arching rows, starting just posterior to choanae, curving postero-medially towards center of roof of mouth; rows of vomerine teeth not in contact medially; maxillary teeth numerous (29/31), present in two long arching single rows on the opposite/outer margins of the upper mouth, out of maxillary bones; premaxillary teeth relatively few (3), present as a short row at anterior tip of upper mouth, out of premaxillary bones. Choanae moderately large, teardrop shaped, with narrow groove emerging from each opening laterally, curving posteriorly beneath eye. On the medial roof of the mouth, lying posterior to the vomerine tooth rows, there is a triangular patch of paravomerine teeth, which are numerous.
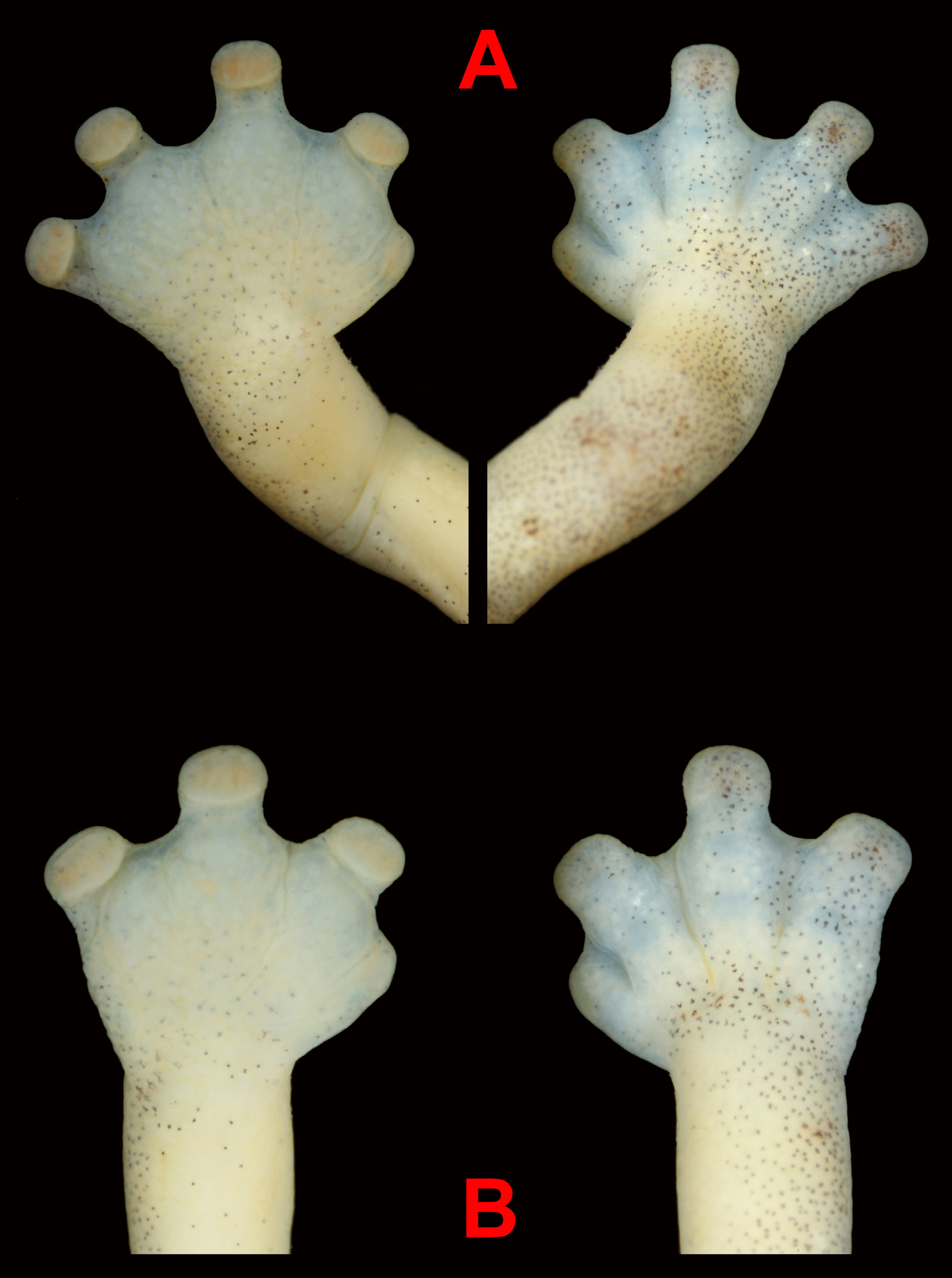
Arms relatively short (FLL 8.3 mm, 20.5 % of SL), with forearm slightly hypertrophied compared to upper arm. Hands moderate-sized (HaL 2.7 mm, 30.7 % of VGS; HaW 3.0 mm, 51.7 % of VGS). Fingers II, III, and IV short and robust with truncate tips nearly equal in width (WF3 0.7 mm); terminal pads present on ventral tips of fingers II, III, and IV; Finger I not protruding beyond interdigital webbing; fingers II and IV with only distal phalanx free of webbing (LF2 0.6 mm), Finger III appearing to have at least 1.5 phalanges free of webbing (LF3 0.9 mm). Palmar surface smooth overall, but with evident dermal creases extending centrally from margins of interdigital webbing. Dorsal surfaces of all fingers with well defined interdigital grooves extending from margins of webbing to metacarpal region. Interdigital webbings on the hands are moderate to extensive, with distal margins of webbing concave in outline. Estimated webbing formula of right hand: I 0- – 1 1/ 2 II 1 1/2 – 2- III 2- – 1+ IV. Relative lengths of fingers on right hand I <II <IV <III ( Fig. 5 View FIGURE 5 B).
Legs relatively short (HLL 8.7 mm, 21.5 % of SL), with little perceivable difference between thickness of upper and lower leg. Feet moderate-sized (FoL 3.3 mm, 37.5 % of VGS; FoW 4.1 mm, 70.7 % of VGS). Toes II, III, IV, and V short and robust with truncate tips nearly equal in width (WT3 0.7 mm), Toe III slightly longer than others (LT2 0.5 mm, LT3 1.0 mm); well-defined terminal pads present on ventral tips of toes II, III, IV, and V, weakly defined and smaller pad present on ventral tip of Toe I; tip of Toe I barely protruding beyond margin of interdigital webbing; toes II and V with only distal phalanx free of webbing, Toes III and IV appearing to have at least 1.5 phalanges free of webbing. Plantar surface smooth overall, but with evident dermal creases extending centrally from margins of interdigital webbing. Dorsally all digits of feet well defined with interdigital grooves traveling inward from webbing margins to metatarsals. Interdigital webbing on feet moderate to extensive, with the distal margins concave in outline. Estimated webbing formula of right foot: I 0- – 1 1/ 2 II 1 – 2- III 2 – 1 1/ 2 IV 1 1/ 2 – 1+ V. Relative lengths of toes on right foot I <II <V <IV <III ( Fig. 5 View FIGURE 5 A).
Body smooth, subcylindrical (wider than high) in cross section, relatively slender (TW 6 mm). Adpressed limbs separated by 4.5 costal folds, with 13 weakly discernable costal grooves between axilla and groin; costal grooves most visible on ventral and lateral portions of the body. Slight middorsal depression traveling longitudinally along length of body, starting at base of head, and becoming indiscernible on anterior third of tail length. Slight constriction at base of tail, between hind limbs and posterior edge of cloacal opening (body 5.2 mm in width at anterior margin of hind limbs, 4.1 mm at midsection of cloacal opening, and 4.4 mm just posterior to cloacal opening). Tail long, cylindrical in cross section, evenly tapering to pointed terminus; 27 caudal grooves discernible on anterior 3/4ths of tail, no grooves discernible on posterior 1/4th of tail.
Skin on all surfaces of head, body, limbs, and tail smooth.
Coloration in life. The holotype was kept alive in captivity for six months by BK to allow for natural history observations prior to being euthanized and preserved. During the months in captivity only very minimal metachrosis was witnessed, being limited to minor tonal shifts in the intensity of the yellow ground color of the head, body, limbs, and tail, and the shift in the flanks and limbs from light yellow to light to medium brown. The tonal shift of the background coloration of the dorsum of the holotype varied from Pale Greenish Yellow (86) to Medium Greenish Yellow (88). The only contrasting coloration on the dorsal surfaces of the head, body, or tail was the middorsal stripe, which started out as a pair of dark brown lines at the posterior/inner margins of the orbits and converge to meet as a single middorsal stripe just posterior of the gular fold, traveling along the mid-dorsum of the body and initiating a curve laterally towards the right side at about the anterior margin of the cloacal opening and connecting to the lateral line on the right side of the body just posterior of the vent ( Fig. 6 View FIGURE 6 A). The middorsal stripe consisted of a mixture of fine pigments between Brick Red (36) to Warm Sepia (40). Scattered evenly throughout much of the ventral and dorsal surfaces of the head, body, limbs, and tail were minute dark melanophores, either in the form of very fine dots or a very fine reticulation; these dark melanophores were visible under magnification.
The highest degree of metachrosis was observed on the lateral margin of the head, body, tail, and limbs, where there was either a thin dorsolateral line starting at the posterior margin of the eyes and extending along the body and onto the tail nearing the tip, or at times appearing as a wider band of pale brown that started at the posterior margin of the nares, extended along the canthal ridge to the anterior corner of the eye, and continued as a wider, better defined pale brown band from the posterior corner of the eye, extending posteriorly along the body and onto the tail almost to the tip; when the wider lateral band was present there also was a pale brown coloration on the dorsal surfaces of the limbs ( Fig. 7 View FIGURE 7 ). The thin lateral lines varied with metachrosis from Brick Red (36) to Warm Sepia (40), indistinguishable in color from that of the mid-dorsal line; when present, the thicker lateral bands and dorsal surfaces of the limbs were Orange-Rufous (56) to Amber (51).
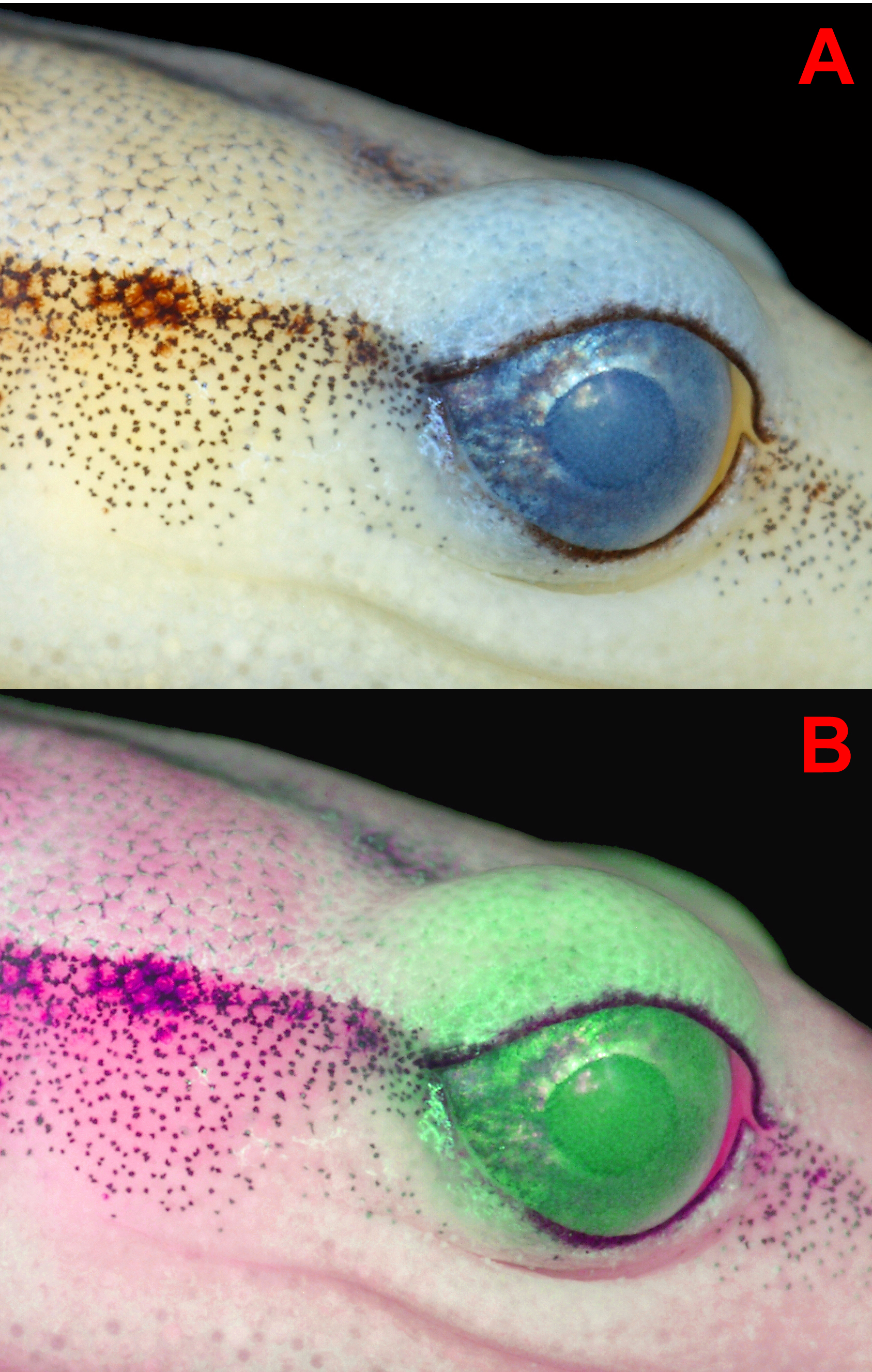
Light iridescent green chromatophores were observed scattered throughout the intense yellow dorsal surfaces of the head, body and tail, and at times resulted in an overall greenish hue to those surfaces; these pigments were especially concentrated and more consistently observed on the eyelids ( Fig. 8 View FIGURE 8 ). When the holotype was first discovered in the field it was immediately noteworthy due to the strong greenish-yellow tone of the dorsum.
The hands and feet typically had a Trogon Yellow (81) coloration, but at times would also present scattered concentrations of Orange-Rufous (56) pigmentation on the proximal dorsal region. The tips of the digits would also vary in coloration from Trogon Yellow (81) to Spectrum Orange (9).
The iris had a golden background color with darker pigments in the form of fine to moderate-sized spots; the dark spotting of the iris was Brick Red (36) to Warm Sepia (40). Additionally, there were also often heavy concentrations of Chartreuse (89) pigments in the iris; the concentration and intensity of the chartreuse pigments were subject to metachrosis.
The ventral skin of the head and body was uniformly translucent yellow, ranging in tone from Cream Color (12) to Light Orange Yellow (77); no contrasting lighter or darker pigmentation in the form of spots or other markings was ever observed ( Fig. 6 View FIGURE 6 B). Longitudinally, along the mid-ventral line, some scattered concentrations of iridophores were observed beneath the skin. Beneath the skin at the midventral gular fold, lying principally posterior of the gular fold, the bright red corpus of the heart was visible. The heart appears to lack pigmentation in the pericardial sac, such as iridophores, thus the red coloration is caused by oxygenated blood. At times heavy concentrations of blood red capillaries were observed throughout the ventral surfaces of the head, body and limbs.
Coloration in ethanol. After three years in ethanol (70%), overall bright yellow coloration has faded to Light Buff (2) and Pale Pinkish Buff (3). The only noticeable contrasting colors on the entire specimen are the dark middorsal stripe, lateral stripes, and minute melanophores scattered throughout the skin surfaces, either in the form of tiny dots or as extremely fine reticulations; the minute melanophores are best visible under magnification. The middorsal stripe and lateral stripes are all constituted by diffuse Pratt’s Rufous (72) pigmentation. The minute melanophores are Dark Grayish Brown (284). In the external layer of the skin above the eyes there is a contrasting pale bluish-white hue, which is likely in relation to the green iridescent pigments that were visibly concentrated in this region in life. Upon close inspection, this bluish-white hue is definitely present as a type of pigmentation in the dermal tissue and not reflective artifact from any pale coloration on the dorsal surface of the orbit itself ( Fig. 9 View FIGURE 9 ).
Measurements (in mm), tooth counts, limb interval, and percentages of the holotype. SL 40.5; Tal 55.6; TL 96.1; ShW 5.6; HeW 5.8; NeW 5.2; EW 2.1; SnL 1.8; JSL 4.1; LGFS 9.0; LNH 0.09; RNW 0.03; IND 1.6; NLP 0.7; ICD 3.2; HLL 8.7; FLL 8.3; TW 6.0; VGS 8.8; FSL 10.6; UHL 5.9; AGL 23.5; VL 3.0; HaW 3.0; HaL 2.7; LF2 0.6; LF3 0.9; WF3 0.7; FoW 4.1; FoL 3.3; LT2 0.5; LT3 1.0; WT3 0.7. Limb interval 4.5. Number of teeth PMT 3, MT 29/31, VT 14/18. Measurements in related percentages: VGS/SL 21.7 %; IND/HeW 27.6 %; AGL/SL 58 %; HeW/SL 14.3 %; Hew/AGL 24.7 %; SnL/ HeW 31 %; LNH/HeW 1.6 %; LNH/SL 0.2 %; RNW/HeW, 0.5%; RNW/SL 0.001 %; HLL/SL 21.5 %; FLL/SL 20.5 %; HaL/VGS 30.7 %; FoL/VGS 37.5 %; Haw/HeW 51.7 %; FoW/HeW 70.7 %; LT3/HeW 17.2 %; LT3/FoW 24.4 %.
Etymology. The name “ aurae ” is in dedication to Aura Reyes, the wife of BK, who co-discovered the holotype and has supported and encouraged BK’s research and conservation efforts with the amphibians of Costa Rica for many years, this in addition to her own contributions to increase the knowledge of Costa Rica’s amphibians made possible through dedicating her time to accompanying BK on numerous field trips, many of which consisted of enduring prolonged periods of cold conditions while being soaking wet within the cloud forests of Costa Rica to search for elusive anuran and caudate species. The name also alludes to the Latin aureus, meaning golden, for the yellow coloration the holotype possessed in life.
Habitat and natural history observations. The site where Bolitoglossa aurae was found is cloud forest, with an abundance of bryophytes and other epiphytes covering the arboreal structures. Bolitoglossa aurae was discovered within moss and decomposing organic material surrounding and intertwined in a root mass of a large orchid attached to a thin rotten tree that was still standing in the forest understory. The rotten tree fell to the ground as soon as a light amount of pressure was applied to it. The orchid, within which the salamander was hiding, had been growing at approximately 3 meters above the ground. Bolitoglossa aurae was found during a diurnal exploration. When the holotype was discovered it had a very evident and impressive greenish hue to the entire dorsal surface, a tone and intensity that was never witnessed again within the six months it was kept alive in captivity. It is possible that the green tone that was observed the day the holotype was collected was not seen again due to the individual being housed in a terrarium with a thick loose and humid layer of dead sphagnum moss, that was pale tan in color. Had the holotype of B. aurae been housed in a terrarium with an abundant supply of live green moss it is possible that the green hue observed when collected might have been observed again.
Inasmuch as Bolitoglossa aurae is only known from a single specimen fortuitously found curled up within an orchid root mass during the day, any assumptions about its natural history are speculative. With that said, however, it is probable that this species is given to living within thick moss mats and other epiphytic growth in arboreal habitats in the cloud forest.
The holotype was kept alive in captivity for six months prior to being euthanized and preserved; it was housed in a small terrarium (50 cm x 30 cm x 25 cm). The enclosure had a thick bed (ca. 10 cm thick) of dead sphagnum moss; no other structures were present in the enclosure to facilitate easy observation of the holotype and its activity and behavior. The individual showed a definite nocturnal behavior, often being observed crawling on the surface of the sphagnum moss or even the walls of the enclosure at night. During the day the holotype would burrow deeply within the loose moist sphagnum moss, only emerging after dusk. The holotype was fed a diet consisting of wild fruit flies (Family: Drosophiliidae). The holotype adapted very well to captivity, maintaining a relatively robust body and tail during the time it was in a terrarium, and would aggressively feeding on numerous fruit flies immediately following their introduction to the enclosure.
Distribution. Bolitoglossa aurae is known only from a single site within the Tropical Premontane Rain Forest life zone ( Holdridge 1967) along the mid-elevation slopes of northeastern Cordillera de Talamanca, in the vicinity of Moravia de Chirripó, ca. 1300 m ( Fig. 1 View FIGURE 1 ).
Remarks. With the addition of Bolitoglossa aurae , the number of salamanders known from Costa Rica is now elevated to 50, of which 27 belong to the genus Bolitoglossa . The relationships within Bolitoglossa were revised by Parra-Olea et al. (2004), who defined subgenera and species groups; however, all the species currently associated with the B. robinsoni clade ( Bolaños & Wake 2009; Boza-Oviedo et al. 2012; Hertz et al. 2013) were described after the efforts by Parra-Olea et al. (2004). All known species of the B. robinsoni clade have been found at remote sites in the Cordillera de Talamanca in eastern Costa Rica and Cordillera Central of western Panama; we propose that the members of this clade be recognized distinctly as the Bolitoglossa robinsoni Species Group, currently containing four species: B. aurae , B. aureogularis , B. jugivagans , and B. robinsoni ( Fig. 10 View FIGURE 10 ).
Given the large expanses of remote and unexplored forest along the Caribbean slopes of the Talamancan mountains in eastern Costa Rica and northwestern Panama, it is likely that in future explorations more information will be made available regarding the distributional ranges of the members of this group, this in addition to the potential discovery and description of additional species. Furthermore, it is important evaluate the taxonomic status of all populations currently considered to belong to B. robinsoni . Bolitoglossa robinsoni was described from a type series of four individuals (holotype and two paratopotypes from Cerro Echandi, and one paratype from Cerro Burú), but Bolaños & Wake (2009) included additional referred specimens from four other sites in the Cordillera de Talamanca as tentatively belonging to this taxon. Bolaños & Wake (2009) noted that the different populations represented by their referred materials potentially might represent separate undescribed species. The DNA sequence of B. robinsoni that was included in our molecular analysis is from one of these dubious populations, Valle del Silencio. Nevertheless, as this was the only sequence available to represent this species as currently recognized, we included it for tentative comparison purposes. In order to obtain more clarity surrounding the actual taxonomic status of certain populations currently assigned to B. robinsoni , topotypic DNA sequences are needed.
The genetic distances among members of the Bolitoglossa robinsoni species group are relatively low in comparison to those suggested by Fouquet et al. (2007) for Neotropical frogs, in which those authors recommend 3% 16S divergence as threshold to define candidate species. Nevertheless, this threshold possibly underestimates the real diversity of Neotropical salamanders. In a recent phylogenetic study of the minute salamanders of the genus Thorius from the Mexican Highlands, two mitochondrial and two nuclear genes efficiently defined the species within the genus, however the 16S divergence among several species were equal or lower than 2% ( Rovito et al. 2013). Despite the relatively narrow genetic distances among the members of the B. robinsoni species group we are confident that these distances are significant, especially when combined with their geographic proximities and robust phenotypic characteristics that define each species even further. Other members of the genus Bolitoglossa from within Costa Rica are also known to have similar narrow genetic distances among congeners, one example of a species well recognized for its phenotypic distinctness, but presenting a relatively low genetic divergence among some of its congeners (as low as 2.0 % 16S) is Bolitoglossa splendida Boza et al., 2012 . Genetic distances of less than 2.5 % are actually common among several members of the Bolitoglossa subpalmata species group within Costa Rica (Boza et al. 2012), and despite these narrow genetic distances, the species of this group are well recognized as being distinct.
| UCR |
University of California |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |