Stiphodon multisquamus Wu & Ni, 1986
|
publication ID |
https://doi.org/ 10.5281/zenodo.5384784 |
|
publication LSID |
lsid:zoobank.org:pub:5A6E3EA2-3C26-4F4D-B14D-839C557762D7 |
|
persistent identifier |
https://treatment.plazi.org/id/03F74959-FFF5-D054-FF34-F971A992F889 |
|
treatment provided by |
Valdenar |
|
scientific name |
Stiphodon multisquamus Wu & Ni, 1986 |
| status |
|
Stiphodon multisquamus Wu & Ni, 1986 View in CoL
( Figs. 1–7 View Fig View Fig View Fig View Fig View Fig View Fig View Fig ; Table 1)
Stiphodon elegans multisquamus Wu & Ni, 1986: 422 View in CoL (type locality: Lingshui River , Hainan Island, China).
Stiphodon multisquamus: Wu, 1991: 495 View in CoL (Hainan Island); Wu, 2008: 729 (Hainan Island); Nip, 2010: 1238 (Guangdong, China); Maeda & Saeki, 2013: 216 (Okinawa Island, Japan).
Stiphodon aureorostrum Chen & Tan, 2005: 238 View in CoL (type locality: Pulau Tioman , Malaysia).
Material examined. Vietnam: ZRC 50267, 12 males (37.2–64.0 mm SL) and 12 females (37.1–51.4 mm SL), Suoi Mo, Hoa Vang District , Da Nang Province, coll. H. H. Tan ,
A. D. Tran et al., 27 February 2005 ; URM-P 48179, 48181, 2 females (29.8–33.8 mm SL), Suoi Mo , Hoa Vang District, Da Nang Province, coll. K. Maeda & H. D. Tran, 12 January 2013 ; URM-P 48199–48207, 4 males (41.7–61.3 mm SL) and 5 females (40.7–51.9 mm SL), Suoi Hoa , Hoa Vang District, Da Nang Province, coll. K. Maeda & H. D. Tran, 13 January 2013 ; URM-P 48214–48216, 48218–48221, 48224, 48225, 6 males (31.9–43.2 mm SL) and 3 females (32.4–37.4 mm SL), Suoi Mo , Hoa Vang District, Da Nang Province, coll. K. Maeda & H. D. Tran, 14 January 2013 . Malaysia: ZRC 45409, 2 females (51.7–58.3 mm SL, paratypes of S. aureorostrum ), Sungai Keliling , Pulau Tioman, coll. H. H. Tan, 25 June 1999 ; ZRC 45410, 1 male (45.4 mm SL, paratype of S. aureorostrum ), Sungai Keliling , Pulau Tioman, coll. P. K. L. Ng et al., 24 June 1999 ; ZRC 46414, 1 female (42.4 mm SL), Sungai Keliling , Pulau Tioman, coll. H. H. Tan, June 1999 ; ZRC 54194, 3 males (43.4–44.6 mm SL) and 2 females (42.6–44.0 mm SL), Sungai Keliling , coll. H. H. Tan & B. W. Low, 16 July 2013 .
Diagnosis. Second dorsal fin usually with one spine and nine soft rays; pectoral fin usually with 15 or 16 soft rays; first dorsal-fin spines in male elongate and tip of the longest spine extending far beyond the second dorsal-fin origin. Premaxilla with 43–66 tricuspid teeth; dentary with 44–67 horizontal teeth. Scales on occipital region and anterior nape significantly smaller than lateral scales on trunk and tail. Scales extending anteriorly to nape or posterior part of occipital region in male, usually extending anteriorly to middle or posterior part of occipital region in female. Predorsal midline with fewer scales than either side of midline, and often naked in male. Nine to 11 dusky, transverse bars laterally on trunk and tail; two or three conspicuous, light-coloured, transverse bars on occipital region and nape; pectoral-fin rays with fine black spots. In male, lateral side of head often bluish; distal part of first and second dorsal fins orange in life. In female, second dorsal-fin spine and soft rays often with two to four black spots; tip of first and second dorsal fins with reddish markings in life; anal fin with a submarginal black line; caudal fin with black spots forming transverse bars.
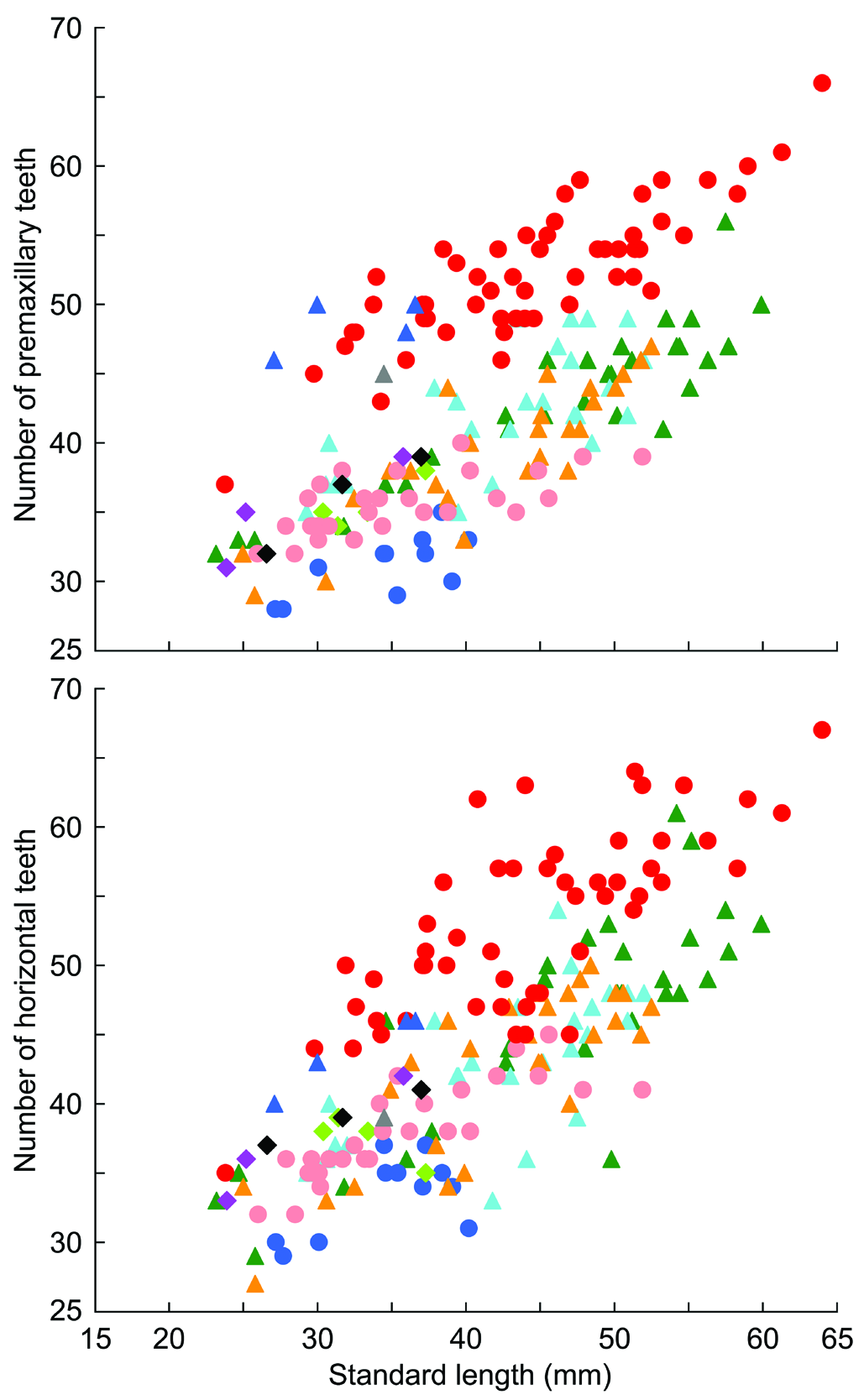
Description. Morphometric measurements given in Table 1. Body elongate, cylindrical anteriorly and somewhat compressed posteriorly. Head somewhat depressed with a round snout protruding beyond upper lip. Anterior nostril tubular and short, posterior nostril not tubular. Mouth inferior with upper jaw projecting beyond lower jaw. Upper lip thick and smooth with small medial cleft. Premaxillary teeth 43–66, fine and tricuspid. Dentary with canine-like symphyseal teeth (number of teeth one to five in male, usually one to three in female; symphyseal teeth absent on right only of one female) and row of 44–67 unicuspid horizontal teeth enclosed in fleshy sheath. Larger individuals have more premaxillary and horizontal teeth than smaller individuals ( Fig. 2 View Fig ). Urogenital papilla rounded in male, rectangular with small projection at each corner of posterior edge in female.
First dorsal fin with six spines (one specimen with five spines); second dorsal fin usually with one spine and nine soft rays (two specimens with one spine and ten soft rays). In female, first dorsal fin almost semicircular with second, third, or fourth spine longest; in male, second to sixth spines elongate (usually fourth spine longest) and membranes sometimes notched between third and fourth, between fourth and fifth, or between fifth and sixth spines, but spines not filamentous ( Fig. 3 View Fig ). Posteriormost point of first dorsal fin of male (tip of fourth and/or fifth spine) extending to base of second to fifth soft ray of second dorsal fin when depressed. Anal fin with one spine and 10 soft rays. In female, first or second soft rays longest in second dorsal fin, and second or third soft rays longest in anal fin; in male, posterior rays longer than anterior rays in those fins (last ray and/or penultimate ray usually longest). Caudal fin with 17 segmented rays, including 13 branched rays, posterior margin rounded; caudal fin relatively larger in male than in female (caudal-fin length 24–28% of SL in male, 20–23% of SL in female). Pectoral fin with 14 (n=5), 15 (n=29), or 16 (n=10) rays. Pelvic fin with one spine and five soft rays; pelvic fins joined together to form strong, cup-like disk with fleshy frenum.
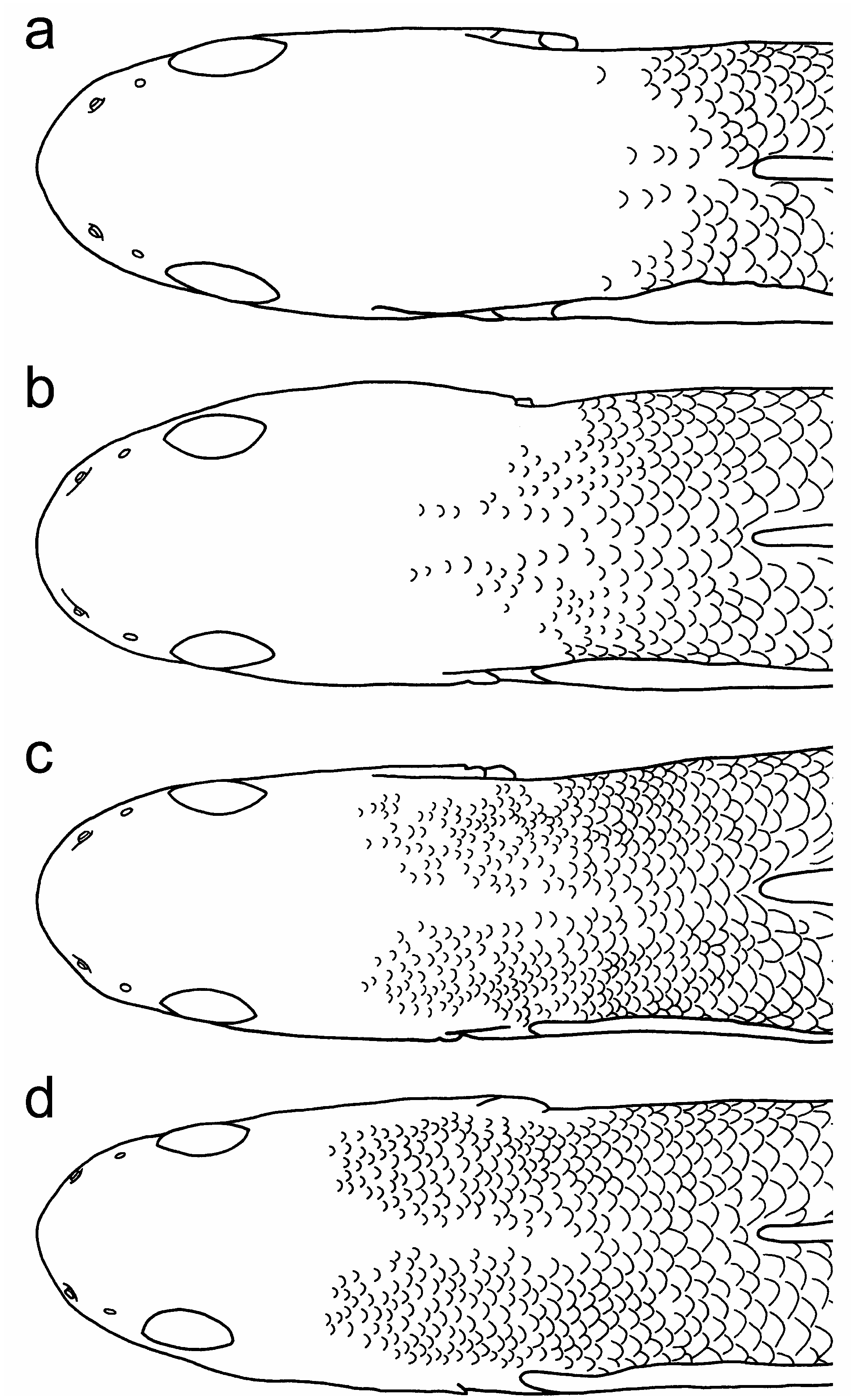
Scales in a longitudinal series 31 (n=2), 32 (n=1), 34 (n=8), 35 (n=26), 36 (n=13), 37 (n=2), 38 (n=1); scales in a transverse series 10 (n=5) or 11 (n=48); circumpeduncular scales 15 (n=2), 16 (n=48), 17 (n=3). Ctenoid scales covering tail and lateral and dorsal sides of posterior trunk. Belly and lateral side of anterior trunk (behind pectoral-fin base) covered by cycloid scales. Scales on nape and occipital region differing between male and female. In male ( Fig. 4a, b View Fig ), scales extended anteriorly to posterior or anterior part of nape or posterior part of occipital region; predorsal midline naked or scaled only on nape. In female ( Fig. 4c, d View Fig ), scales extended anteriorly to middle or posterior part of occipital region (one female without scale on occipital region); whole predorsal midline sometimes naked but posterior part of nape usually scaled. All scales on occipital region and nape cycloid and scales on occipital region and anterior nape notably smaller than lateral scales on trunk and tail. Cycloid scales also occurring on first and second dorsal-fin bases, anal-fin base, caudal-fin base, and proximal part of caudal fin.
Cephalic sensory pore system invariably A´, B, C, D(S), F, H´, K´, L´, N´, and O´. Oculoscapular canal interrupted between pores H´ and K´. Cutaneous sensory papillae developed over dorsal, lateral, and ventral surface of head.
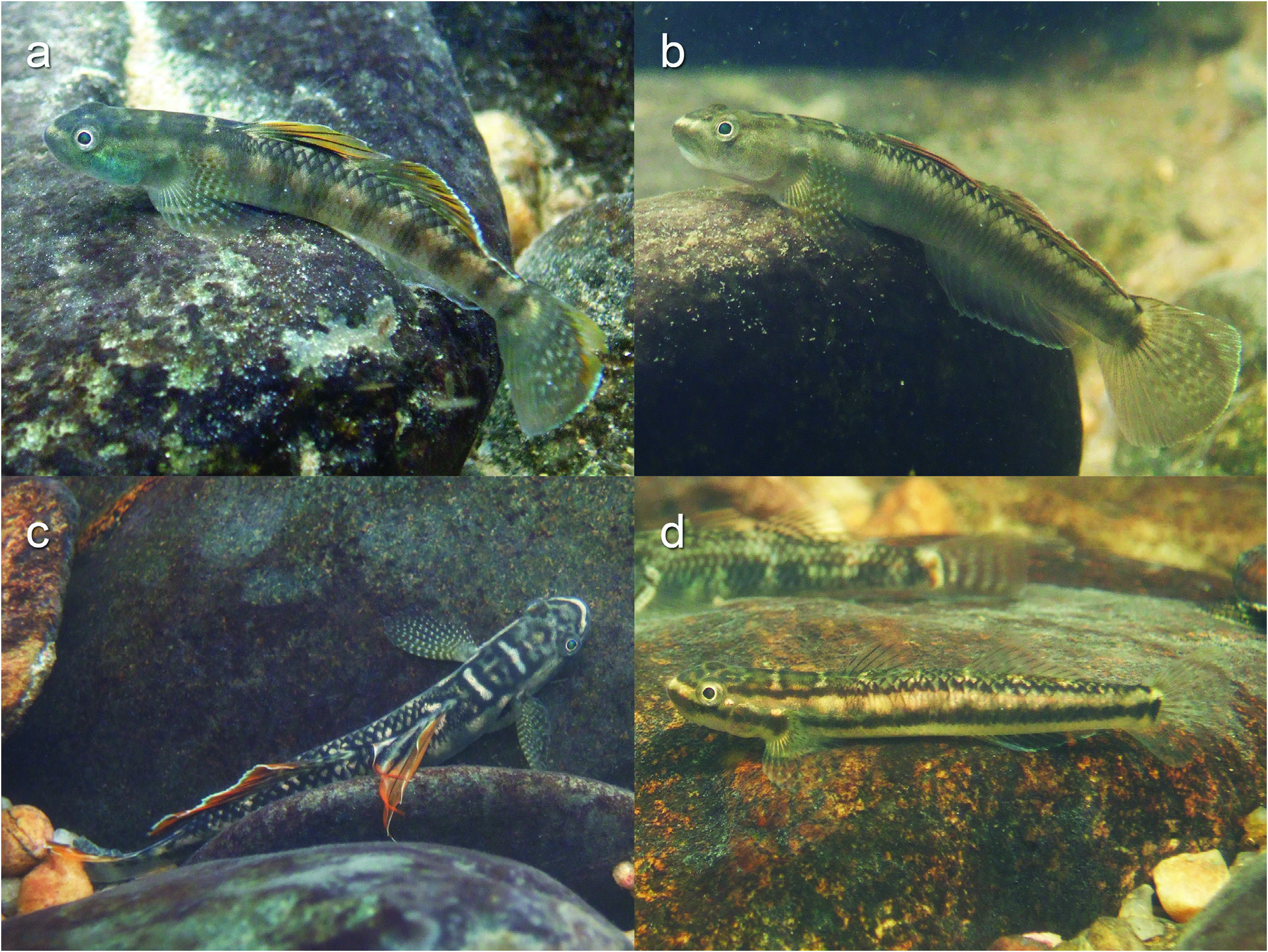
Colour in preservative. Sexual dichromatism well developed. In male ( Fig. 1a, b View Fig ), dorsal background dusky, lateral sides light brown, ventral side pale grey; nine to 11
dusky transverse bars laterally on trunk and tail; width of bars and intervals between them differing among bars, and number and arrangement of bars often differing between right and left sides of body; head and pectoral-fin base dusky; two or three conspicuous pale-brown or cream-coloured transverse bars dorsally on occipital region and nape. First dorsal-fin spines black distally and dusky proximally; membranes transparent distally and dusky proximally. Posterior half of first dorsal fin with black blotch. Second dorsal-fin rays dusky; membranes transparent distally and partly dusky proximally. Anal fin dusky. Caudal fin dusky with translucent spots often forming two to 11 transverse bars on middle part of fin; distal margin of upper part of caudal fin translucent. Pectoral-fin membranes translucent; rays with fine black spots, number of spots on longest rays (seventh and/or eighth ray) eight to 16. Middle to proximal part of pelvic-fin rays, fin membranes, and frenum dusky or blackish, distal margin translucent except for dusky edge around fifth soft ray.
In female ( Fig. 1c, d View Fig ), background of body and head cream coloured; blackish longitudinal band extending from snout and upper lip to below eye and to middle of pectoral-fin base, band continuing from behind pectoral-fin base to posterior end of caudal peduncle through lateral midline; nine to 11 dusky transverse bars laterally on trunk and tail. Dorsum dusky with two or three conspicuous cream coloured transverse bars on occipital region and nape. First dorsal-fin membranes almost transparent, but dusky along black spines. Second dorsal fin bordered by narrow transparent margin; two to four black spots often along each spine and soft ray. Anal fin transparent with black line running near transparent margin. Blackish longitudinal band on caudal peduncle extending to proximal part of caudal fin; caudal fin transparent with black spots forming four to seven transverse bars on dorsal and middle parts. Pectoral-fin membranes translucent; rays with fine black spots, number of spots on longest rays (seventh and/ or eighth ray) four to 11. Pelvic fin translucent, but middle parts of rays, membranes, and frenum often blackish forming a blackish ring in ventral view.
Colour in life. Colour of live males ( Fig. 5a–c View Fig ) variable. Body and fin markings similar to those of preserved specimens, but background yellowish grey to purplish; lateral sides of head yellowish grey to grey, often bluish. Distal part of first and second dorsal fins orange; second dorsal and anal fins with narrow bluish-white edge. Distal part of caudal fin orange or yellowish with narrow bluish-white posterior edge. Pectoral-fin rays with white spots between black spots. Margin of pelvic fin white.
Background of body and head of female ( Fig. 5d View Fig ) cream to light brown; body and fin markings similar to those of preserved specimens; tips of first and second dorsal fins with reddish markings.
Habitat in Vietnam. A population of S. multisquamus was found in Da Nang Province, central Vietnam, where the mountains are relatively close to the coast. This species was very abundant at all three sites, along two tributaries of the Han River at the foot of Ba Na Mountain (1,487 m a.s.l.). Collection sites were located from the middle to upper reaches of the tributaries (34–44 km from the mouth of the Han River), and altitudes were 20–210 m a.s.l. Stiphodon multisquamus was the only sicydiine species found there. Thirty-three other species of fishes were found syntopically with S. multisquamus at these three sites. While S. multisquamus and some other gobioid species are considered amphidromous, all others are freshwater fishes without a marine larval phase ( Table 2).
Remarks. Although the holotype of S. multisquamus has been lost ( Wu, 2008), and we have not examined specimens from the type locality (Hainan Island), females of the Vietnamese specimens examined in the present study match the original description of S. e. multisquamus in Wu & Ni (1986) in all respects, except for the number of scales in a longitudinal series (31–38 in Vietnamese specimens vs. 48 in Wu & Ni, 1986). This difference in scale counts could easily be caused by a difference in counting methods, as discussed in Maeda & Saeki (2013). In fact, Wu (2008) reported 34–35 scales in longitudinal series of two non-type specimens from Hainan. The morphology of the Vietnamese specimens also closely matches the description of four Okinawan specimens ( Maeda & Saeki, 2013) and we are confident that they belong to S. multisquamus , which could be distinguished clearly from all congeners sharing the second dorsal- and pectoral-fin ray counts (one spine and nine soft rays in the second dorsal fin, and usually 15 or 16 soft rays in the pectoral fin) as follows: from S. atropurpureus , S. carisa Watson, 2008 , S. kalfatak Keith, Marquet &Watson, 2007 , S. larson Watson, 1996 , and S. semoni Weber, 1895 by having fine black spots on the pectoral-fin rays (vs. without such spots), pointed, triangular-shaped first dorsal fin in male (vs. semicircular fin, except for S. carisa males with triangular-fin), and a lack of any special structure behind the pectoral-fin base of male (vs. with a white attachment in males); from S. alcedo Maeda, Mukai & Tachihara, 2012a , S. atratus Watson, 1996 , S. imperiorientis Watson & Chen, 1998 , S. martenstyni Watson, 1998 , S. niraikanaiensis Maeda, 2013 , S. ornatus Meinken, 1974 , S. pelewensis Herre, 1936 , S. pulchellus ( Herre, 1927) , and S. weberi Watson, Allen & Kottelat, 1998 by features of predorsal squamation [occipital region is often naked in male, and scales on the occipital region and anterior nape significantly smaller than the lateral scales on the trunk and tail, Fig. 4 View Fig , vs. having larger scales and scaled area extends anteriorly to middle of the occipital region, as Fig. 2 View Fig in Maeda & Tan (2013) and Fig. 2 View Fig in Maeda (2013)], and higher teeth counts ( Fig. 2 View Fig ; but S. martenstyni and S. niraikanaiensis also have higher counts); from S. martenstyni also by having fine black spots on the pectoral-fin rays (vs. without such spots); from S. niraikanaiensis also by lack of black longitudinal band on the second dorsal fin in male (vs. having a broad black band running along the distal margin of the second dorsal fin in male), and having a black line running along the transparent margin of the anal fin in female (vs. no clear markings in female). Stiphodon multisquamus has two or three conspicuous light-coloured transverse bars on the occipital region and nape, which is a useful character to distinguish it from S. maculidorsalis Maeda & Tan, 2013 (having black spots scattering dorsally on the head and trunk), as well as from all other congeners.
Most species of the genus Stiphodon had once been called S. elegans , before Watson (1995a) re-described S. elegans and showed that it is a species actually restricted to Society, Tubuai, and the Samoa Islands in the South Pacific ( Kottelat, 2013). When Wu & Ni (1986) described S. multisquamus as a new subspecies of S. elegans , they actually compared the holotype with “ Stiphodon elegans elegans (Steindachner) recorded from Java ( Wu & Ni, 1986: 303)”. It does not represent true S. elegans , from which S. multisquamus is distinguished by a higher pectoral-fin ray count (usually 15–16 vs. 14), a greater number of teeth ( Fig. 2 View Fig ), and features of predorsal squamation (vs. the nape and posterior half of the occipital region covered by larger scales), as well as by their unique colouration (e.g., S. elegans has clear black and white spots alternately arranged along the first and second dorsal fins in both sexes).
| ZRC |
Zoological Reference Collection, National University of Singapore |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Stiphodon multisquamus Wu & Ni, 1986
| Maeda, Ken, Tran, Hau Duc & Tan, Heok Hui 2015 |
Stiphodon aureorostrum
| Chen I-S & Tan HH 2005: 238 |
Stiphodon multisquamus: Wu, 1991: 495
| Maeda K & Saeki T 2013: 216 |
| Nip THM 2010: 1238 |
| Wu H 2008: 729 |
| Wu H 1991: 495 |
Stiphodon elegans multisquamus
| Wu H & Ni Y 1986: 422 |