Philotheca myoporoides subsp. myoporoides
|
publication ID |
https://doi.org/10.1071/SB22003 |
|
DOI |
https://doi.org/10.5281/zenodo.10974398 |
|
persistent identifier |
https://treatment.plazi.org/id/03F787B6-BD4C-FFB1-FC9A-05F9FF3F870B |
|
treatment provided by |
Felipe |
|
scientific name |
Philotheca myoporoides subsp. myoporoides |
| status |
|
Polyphyly of Philotheca myoporoides subsp. myoporoides View in CoL View at ENA
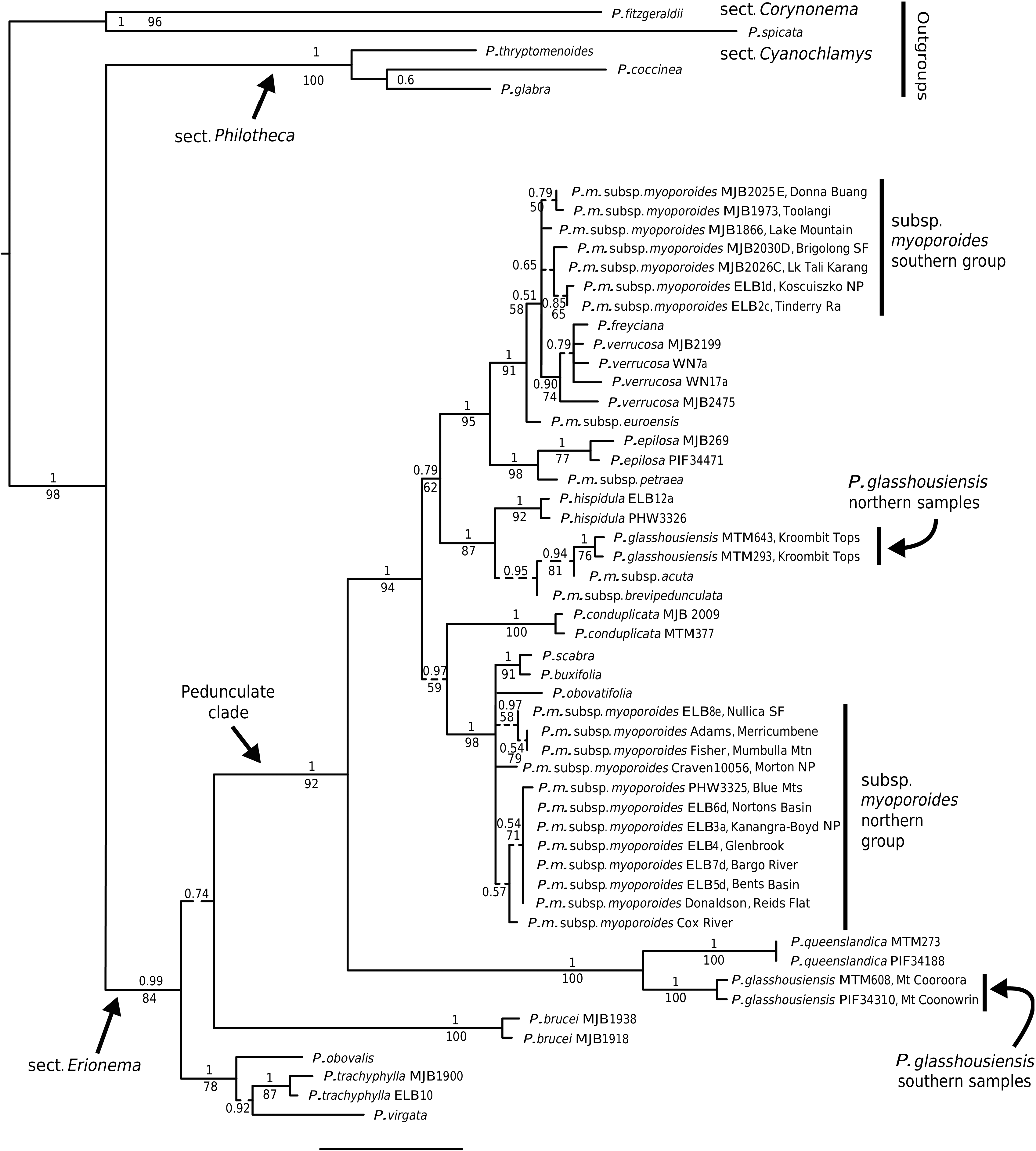
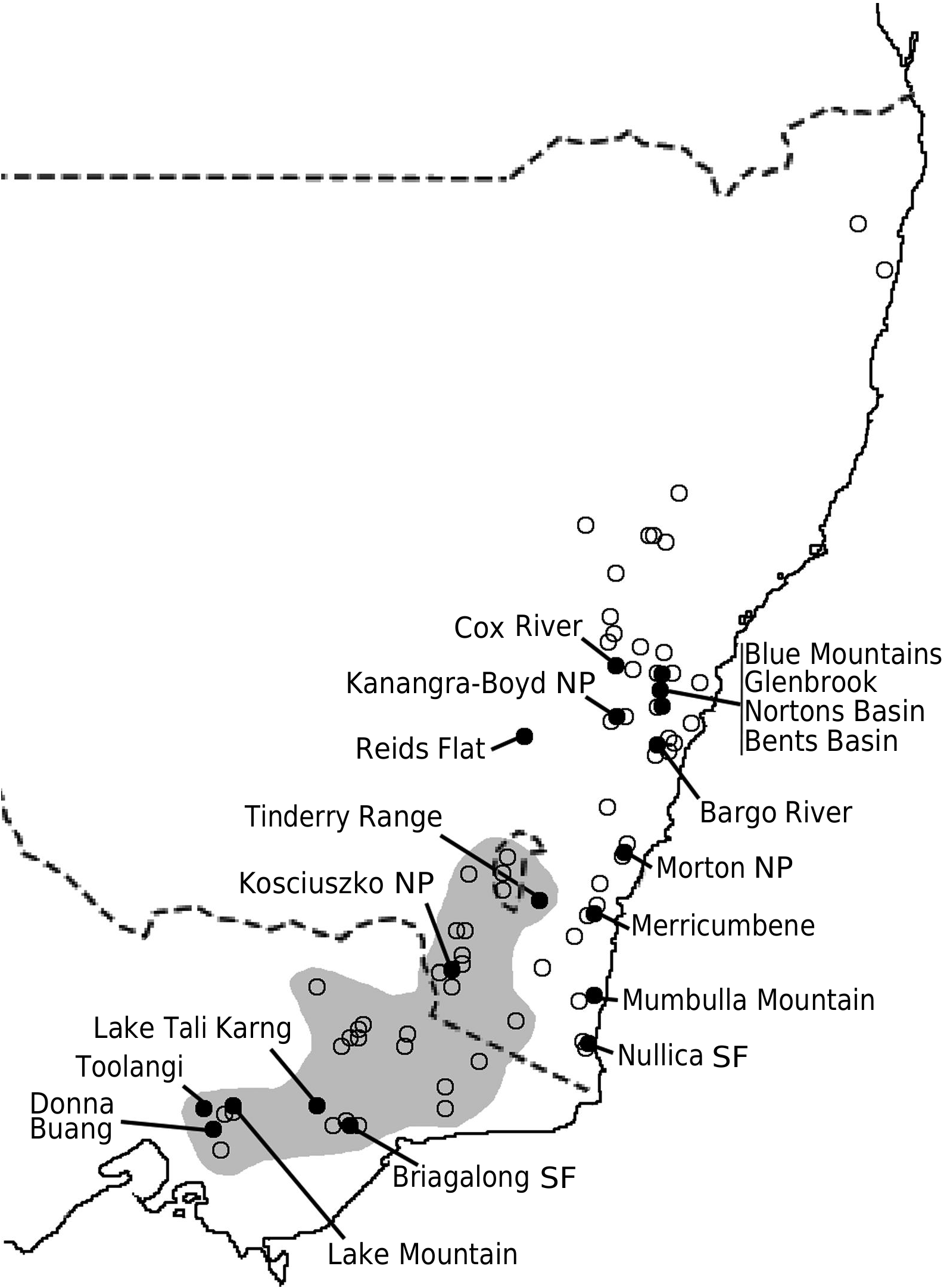
Philotheca myoporoides subsp. myoporoides View in CoL View at ENA is clearly polyphyletic, with samples falling in two well-separated parts of the nrDNA tree ( Fig. 2 View Fig ). These two genetic groups are geographically and ecologically separated and align, at least partly, with morphological variants previously discussed by Wilson (1970, 2013) and Bayly (1998). In Fig. 3 View Fig we have mapped the distribution of the two genetic groups and have also indicated, based on preliminary examination of herbarium material, what we infer to be the geographical ranges of these groups.
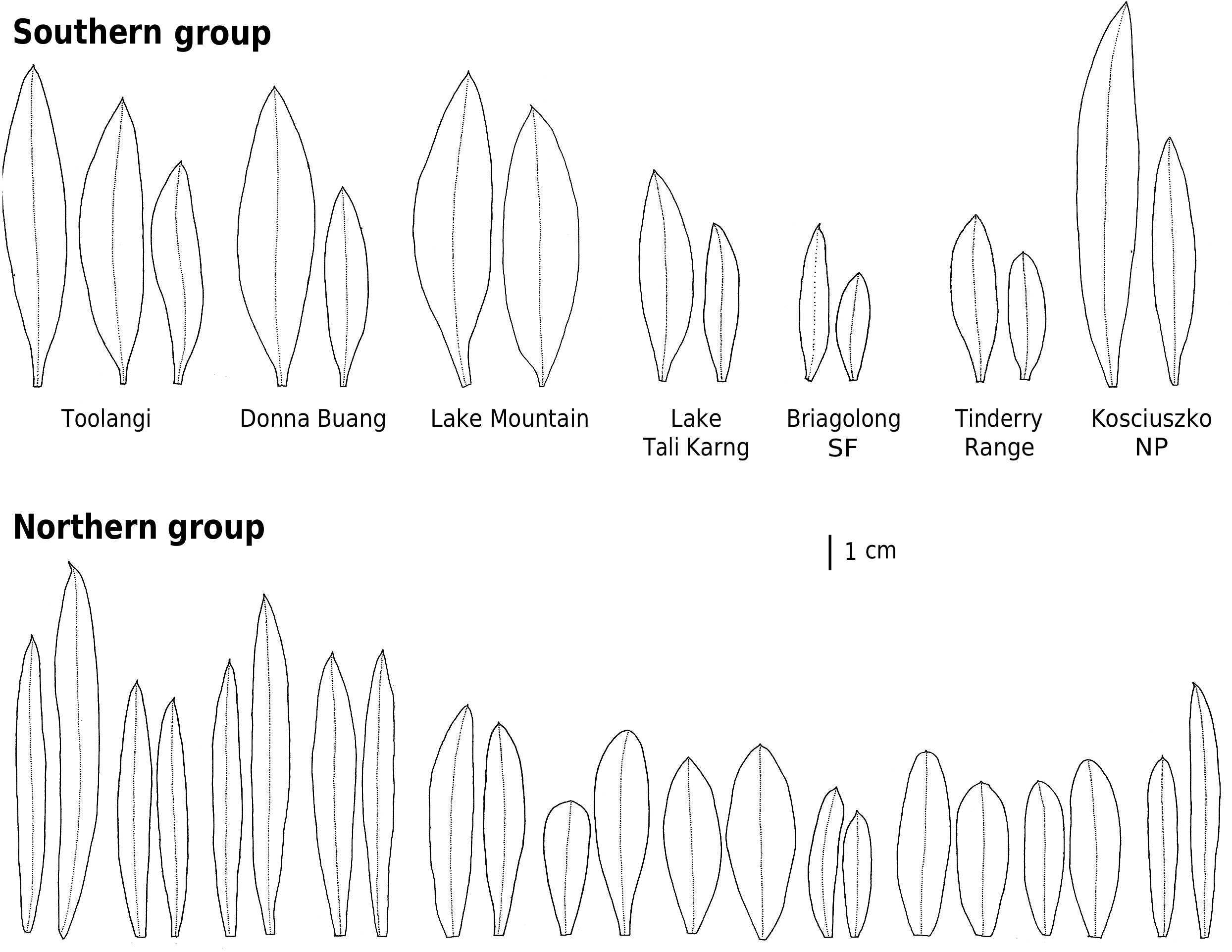
The ‘southern’ genetic group includes the samples from the highlands of Vic., southern NSW and the Australian Capital Territory ( ACT). These typically grow in montane or subalpine forests, commonly in Vic. in forests dominated by Mountain Ash ( Eucalyptus regnans F.Muell. ) or Alpine Ash ( Eucalyptus delegatensis R.T. Baker ), often around granite outcrops but also on other substrates. This group equates to the ‘mountain form’ of subsp. myoporoides View in CoL View at ENA discussed by Wilson (1970, 2013) and Bayly (1998). The earliest species name relating to this group is Eriostemon lancifolius F.Muell. Plants of this group often have leaves that are broader, relative to their length, than those of the ‘northern’ genetic group but are variable in leaf shape and size (e.g. Fig. 4 View Fig ).
The ‘northern’ nrDNA group in subsp. myoporoides View in CoL View at ENA is restricted to NSW and is disjunct from populations of the ‘southern group’ ( Fig. 3 View Fig ). It occurs in a range of habitats, mostly at lower altitudes than populations of the ‘southern group’ and is morphologically variable. The samples in our dataset from the Blue Mountains, Glenbrook, Kanangra–Boyd National Park, Bargo River, Bents Basin and Nortons Basin (all from the Central Coast and Central Tablelands of NSW, along water courses, mostly at low altitudes), morphologically match the type of P. myoporoides and have relatively long, linear leaves ( Fig. 4 View Fig ). Other samples in the ‘northern’ nrDNA group have different leaf tips and the leaf shapes are relatively short and broad. These include the sample from Cox River that morphologically resembles the type of Eriostemon cuspidatus A.Cunn. from the same locality. Other samples with shorter or broader leaves include that from Reids Flat near Bigga in the Central Tablelands and samples from rocky areas in hills and escarpments of the South Coast or Southern Tablelands regions (Merricumbene Forest in Deua National Park, Morton National Park and Mumbulla Mountain). The last group of specimens occurs in localities close to those of P. myoporoides subsp. brevipedunculata (in Deua National Park and nearby areas) in similar upland habitats; although approaching that taxon in leaf dimensions they have larger leaves, longer peduncles and distinct nrDNA from the single sample of subsp. brevipedunculata included here. Nonetheless, the morphological, ecological and genetic distinctiveness of subsp. brevipedunculata in this area could be worthy of further detailed investigation based on more intensive sampling.
The two distinct genetic groups in subsp. myoporoides should be recognised as at least two different species. However, further work is required to clearly circumscribe them. Although we assign herbarium samples to morphological groups (as in the maps in Fig. 3 View Fig ), there is substantial morphological variation within these groups, especially the northern one, and despite the morphological extremes being fairly distinct, there is a lack of clear separation in leaf attributes (or reproductive features) when all specimens are considered.
Also, in the analysis here, neither the ‘southern’ nor ‘northern’ nrDNA groups in subsp. myoporoides was resolved as monophyletic. This could be due to a lack of signal in the ITS and ETS markers or, amongst other hypotheses, it could reflect the presence of multiple taxa in these nrDNA groups. Other species that group closely with the genetic groups of subsp. myoporoides in the nrDNA tree (e.g. P. scabra , P. buxifolia and P. obovatifolia with the ‘northern’ nrDNA group, and P. verrucosa with the ‘southern group’) are morphologically distinct, to the extent that they could not reasonably be treated as conspecific. Further resolution of both genetic relationships and morphological variation in the nrDNA groups of subsp. myoporoides is needed to inform the delimitation of taxa.
| NSW |
Royal Botanic Gardens, National Herbarium of New South Wales |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |