Holopedium atlanticum, Rowe, Chad L., Adamowicz, Sarah J. & Hebert, Paul D. N., 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.179852 |
|
DOI |
https://doi.org/10.5281/zenodo.5614584 |
|
persistent identifier |
https://treatment.plazi.org/id/03F8375C-9D02-FFBC-FF33-A3C9FC97C2B1 |
|
treatment provided by |
Plazi |
|
scientific name |
Holopedium atlanticum |
| status |
sp. nov. |
Holopedium atlanticum View in CoL n. sp.
Synonymy. Individuals from North America previously identified as H. amazonicum should properly be identified as H. atlanticum .
Birge (1918): 693, Fig. 1061b
Pennak (1953): 364–365, Fig. 227d
Brooks (1959): 603, Fig. 27.13
Pennak (1978): 365–366, Fig. 254d
Pennak (1989): 386–387, Fig. 12d
Korovchinsky (1992): 77–78, Figs. 371–373, 375, 377
Etymology. atlanticum refers to the distribution of this species in lakes along the eastern Atlantic seaboard of North America.
Type locality. Moosehead Lake, Maine (45.633º N, 69.683º W). On Hwy ME-6, in close proximity to the town of Moosehead.
Type specimens. Holotype: an ovigerous female in ethanol deposited in the CMN under accession number CMNC 2007-0741 (collection date September 2, 1993).
Paratypes: 10 ovigerous females, preserved in ethanol, deposited in the CMN under accession number CMNC 2007-0742 (collection date September 2, 1993).
Material examined. Other habitats with H. atlanticum are listed in Appendix A.
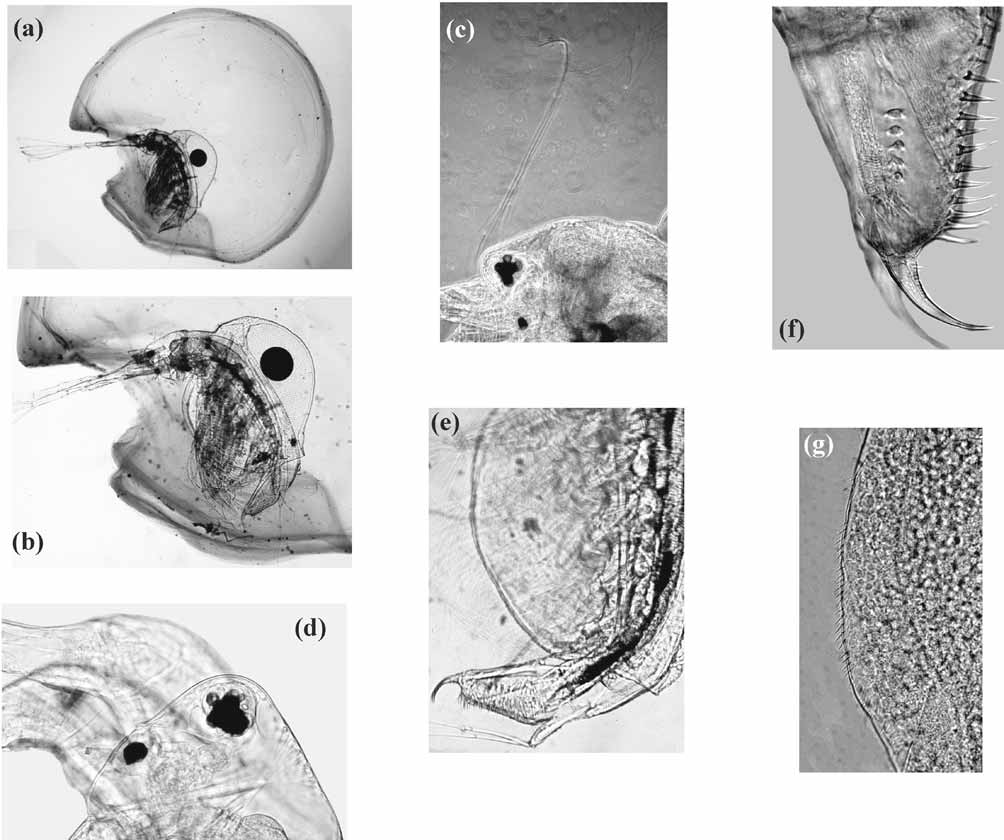
Morphological description. FEMALE. Representative photomicrographs are shown in Fig. 10 View FIGURE 10 . The jelly coat is of the A type, in which the anterior jelly curl arches toward the anterior portion of the jelly coat, and the lateral lobes are undivided (see Montvilo et al. 1987).
Adult carapace lengths range from 0.44–1.01 mm (mean 0.73 mm), while carapace heights range from 0.30–1.06 mm (mean 0.74 mm). The H/L ratios range from 0.68–1.37 (mean 1.00). The ventral carapace margin is ordinarily spinulated posteriorly, but smooth anteriorly. Individuals lacking spinulation along the entire ventral valve margin were encountered.
Anal spine number ranges from 6–11 (mean 8.35). Holopedium atlanticum lacks a basal spine on each postabdominal claw. Each claw ordinarily has a row of denticles running laterally from the base of the claw to its midpoint, although individuals were observed that lacked claw denticulation.
MALE. Males have been found in small numbers in collections from sites in North Carolina in May and June; however, they are typically found in the highest abundance in the autumn ( Hegyi 1973). Males of this species were not examined in this study, and thus detailed morphometrics cannot be presented. However, Hegyi (1973) presented a photograph and brief description of a male Holopedium which, based on distributional data, is probably H. atlanticum .
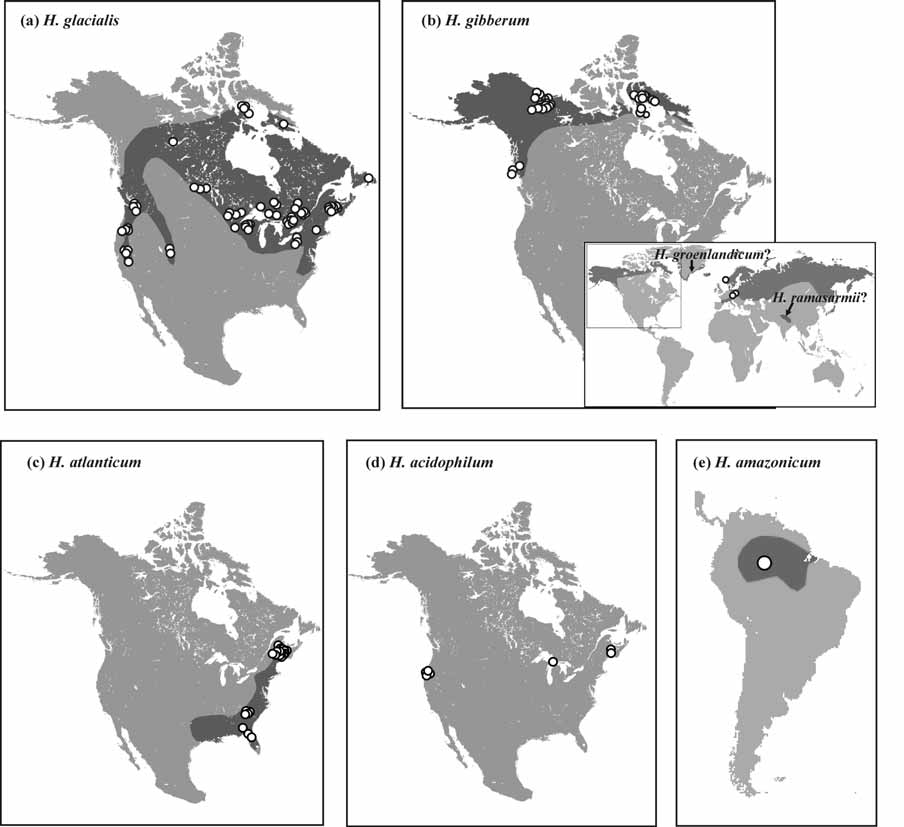
Differential diagnosis. Although H. atlanticum is morphologically indistinguishable from H. amazonicum , these two species have allopatric distributions reducing the likelihood of genetic exchange ( Fig. 4 View FIGURE 4 c,e). Holopedium atlanticum is distinguished from H. acidophilum by the larger size and greater number of anal spines of the latter species. It differs from members of the H. gibberum complex by the absence of a basal spine on either postabdominal claw. Holopedium atlanticum can be biochemically distinguished from H. acidophilum at the Pgm locus, as H. atlanticum produces an enzyme which migrates slower than that of the latter species. COI mtDNA sequence divergence between H. atlanticum and H. amazonicum averages 12.3%, while the divergence between H. atlanticum and H. acidophilum averages 10.6%. Based on current evidence, individuals showing less than 4.8% divergence from a representative COI mtDNA sequence (GenBank AF 245353 View Materials ) belong to H. atlanticum .
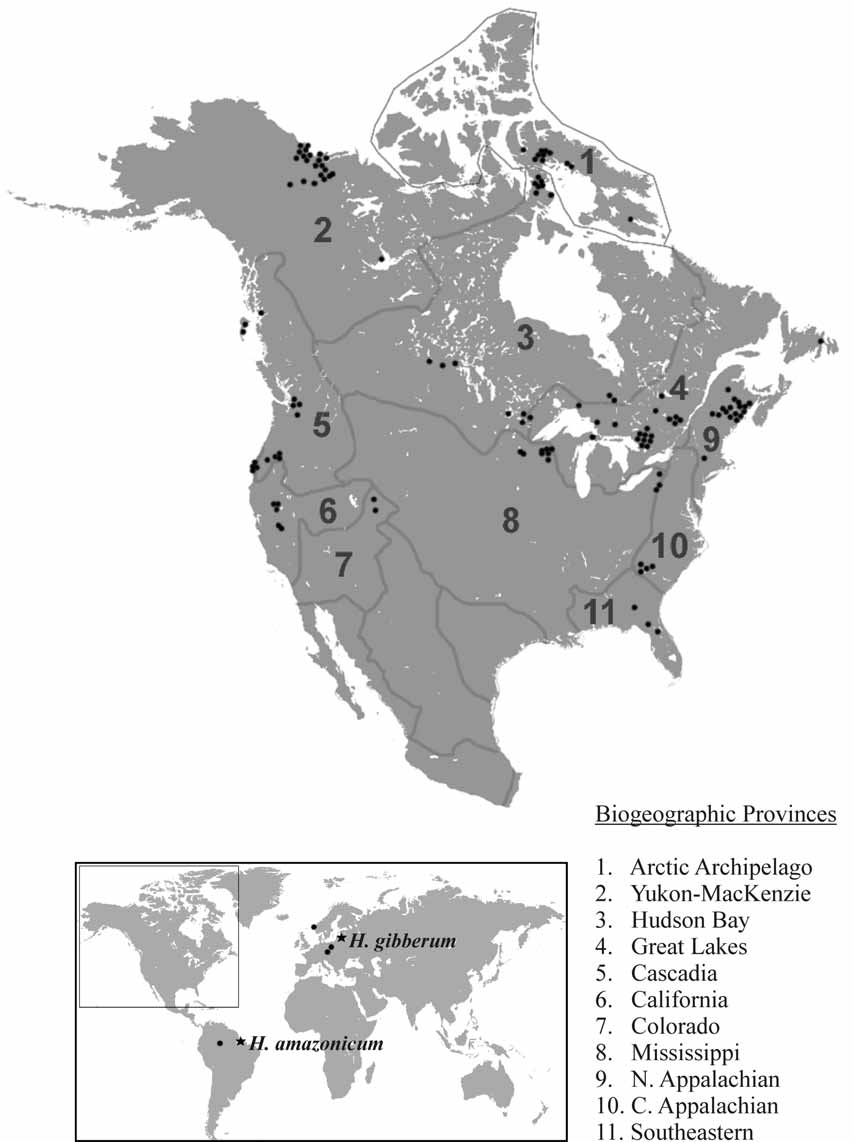
Distribution. H. atlanticum was found along the Atlantic coast of North America from New Brunswick and Maine south to Florida, ( Fig. 4 View FIGURE 4 c). Populations of Holopedium reported by other workers from the southeastern United States are likely also H. atlanticum . Its range overlaps that of H. glacialis in the northeastern USA and southern New Brunswick, where these species occur sympatrically without hybridization. The extent of range overlap with H. glacialis is unresolved by this study, but several workers have identified H. atlanticum (formerly H. amazonicum ) as far north as New Brunswick and H. glacialis (formerly H. gibberum ) as far south as Tennessee and possibly South Carolina ( Coker 1938, Bunting 1970, Hebert & Finston 1997).
Breeding system. Males were not detected in populations collected throughout the summer in this study. In a life history study spanning two years, males were most abundant in early spring and late autumn ( Hegyi 1973). In some southern localities, populations persist throughout the winter. Due to the existence of males, this species likely reproduces by cyclic parthenogenesis, but there is very little allozyme variation, suggesting that either this species engages in sexual reproduction infrequently or that variation has been trimmed due to a population bottleneck.
A note regarding H. groenlandicum and H. ramasarmii
While individuals from Greenland were not included in the present study, the recently described species H. groenlandicum ( Korovchinsky 2005) can purportedly be distinguished from H. gibberum by its “dorsally low shell and jelly envelope, shorter row of valve marginal spinules which are subdivided in groups, and comparatively longer postabdominal claws.” However, shell shape is a highly variable feature, which may be environmentally influenced ( Røen 1962) and can depend upon the locality and presence/absence of fish (CLR pers. obs). The body lengths (0.74 to 1.09mm, mean 1.45mm), carapace heights (0.80 to 1.57mm, mean 1.19mm), and H:L ratios (0.641 to 1.000, mean 0.814) found by Korovchinsky (2005) in the Greenland populations fall within the ranges of values found in H. gibberum and H. glacialis populations in the present study (the preceding ranges and means that were not published in Korovchinsky [2005] were provided to CLR by that author). Jelly coat shape may be influenced by preservation (CLR, pers. obs), and therefore this trait may not be a good feature for diagnosing species. Moreover, the degree of carapace margin spinulation is also a highly variable trait within species (present study), although the discontinuous nature of the spinulation in the Greenland populations is noteworthy. Finally, the length of the postabdominal claws reported by Korovchinsky (2005, his Figure 1 View FIGURE 1 ) is within the range of claw lengths observed for the H. gibberum s.s. populations studied here. Furthermore, the fact that we detected closely related lineages of H. gibberum s.s. in both northern Europe and North America suggests that similar lineages may be found in intervening arctic areas.
Individuals from India were also not included in the present study. Consideration of the differences between either of the species in the H. gibberum complex and H. ramasarmii ( Rao et al. 1998) is not currently possible due to the poor description of the latter species, lacking in detail. Korovchinsky (2004) labeled this species incertae sedis.
We suggest that genetic evidence is required to determine if H. groenlandicum and H. ramasarmii are distinct species or if they are synonymous with described taxa.
| CMN |
Canadian Museum of Nature |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.