Rohanixalus vittatus ( Boulenger, 1887 ), 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4878.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:34C96340-F0F5-440F-AEEB-6AC50F175950 |
|
DOI |
https://doi.org/10.5281/zenodo.4425398 |
|
persistent identifier |
https://treatment.plazi.org/id/03F8BC2E-FF87-FFF5-CBA7-FF71B461985D |
|
treatment provided by |
Plazi |
|
scientific name |
Rohanixalus vittatus ( Boulenger, 1887 ) |
| status |
comb. nov. |
Rohanixalus vittatus ( Boulenger, 1887) comb. nov.
Striped Bubble-nest Frog
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 , 7–13 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 ; Tables 1 View TABLE 1 , 3 View TABLE 3 , 4 View TABLE 4 )
Original name and description. Ixalus vittatus Boulenger, 1887 . Boulenger G. A. 1887. An account of the batrachians obtained in Burma by M.L. Fea of the Genoa Civic Museum. Annali del Museo Civico di Storia Naturale di Genova, Serie 2, 5: 418–424. Type. Lectotype, MSNG 29397. Type locality. “Bhamò” (= Bhamo, Myanmar). Current status of specific name. Valid name as Rohanixalus vittatus ( Boulenger, 1887) comb. nov.
Taxonomic remarks. Rohanixalus vittatus ( Boulenger, 1887) , the type species of the genus, was originally described from “Bhamò” (= Bhamo), based on the collection of Col. M. L. Fea. Although Boulenger (1887) did not provide further specific details concerning the collection locality of this taxon, his understanding of “Bhamo, as used throughout the work [e.g., “Bhamò...(Upper Irawaddy)”; also often clearly distinguishing it from” other localities in “Kakhien Hills” (= Kachin Hills)], is likely to refer to the city Bhamo (not Bhamo district) situated close to the banks of Irrawaddy River, Kachin state in northeastern part of Myanmar. It is also noteworthy that in the same issue as Boulenger’s work (1887), Thorell (1887) more elaborately discussed the voyages of Col. M. L. Fea based on his spider collections, indicating that Fea’s collections from Bhamo were gathered in “Nord della Birmania, luno l’Iravaddi superiore” (= northern Burma, along the Upper Iravaddi) and that “Fra Rangoon e Bham seguendo l’Iravaddi, corrono circa 900 miglia inglesi” (= approximately 900 British miles run between Yangon and Bhamo, following the Iravaddi).
Rohanixalus vittatus is reported to occur widely across South and Southeast Asia. There have, however, been no records of the species from the type locality ever since its original description. Aowphol et al. (2013) reported sequence data referring to this taxon from two districts within the Kachin state (Myitkyina and Putao, both north of Bhamo), and Dewei (= Dawei), Tanintharyi division in southwestern Myanmar. An additional sample from Shan state in eastern Myanmar is also included in the present study. Phylogenetically, all the available samples from Myanmar nest in four distinct lineages, suggesting that R. vittatus is a complex of multiple species (‘ Feihyla vittata View in CoL ’ Group I and Group II as per Aowphol et al. 2013; four lineages in the genus Rohanixalus in the present study, Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ). However, to which of these lineages does the name R. vittatus apply, is questionable. The geographical data for the available Myanmar collections (available at California Academy of Sciences: https://www.calacademy.org/scientists/herpetology-collection) suggests an elevation-linked distribution pattern. The populations from Putao in the northernmost region of Myanmar (‘ Feihyla vittata View in CoL ’ Group I of Aowphol et al. 2013; Rohanixalus cf. shyamrupus in the present study, Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ) are from elevations of ~ 400–650 m asl. These are probably related to R. shyamrupus that was originally described from the neighbouring Arunachal Pradesh state in India. Whereas, the populations from lowland regions of Myitkyina and Dawei of Myanmar form another distinct lineage (‘ Feihyla vittata View in CoL ’ Group II of Aowphol et al. 2013; Rohanixalus vittatus in the present study, Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ), which is likely to be widely distributed based on disjunct records in lowlands of Myanmar, Thailand ( Aowphol et al. 2013), and the Andaman Islands of India (present study). Another population from elevations of> 1500 m asl in eastern Myanmar (present study) potentially represents a third lineage (included in ‘ Feihyla vittata View in CoL ’ Group I of Aowphol et al. 2013; Rohanixalus sp. 1 in the present study, Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ) that nests along with a previously reported Laos sample ~ 770 m asl ( Meegaskumbura et al. 2002). This lineage shows a sister relationship with samples from Yunnan region of China that falls in a moderately high elevation zone (included in ‘ Feihyla vittata View in CoL ’ Group I of Aowphol et al. 2013; Rohanixalus sp. 2 in the present study, Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ).
Thus, based on its original type locality, Bhamo, R. vittatus is considered to be a low elevation species, widely distributed in lowland areas right from northern (Myitkyina district in Kachin state) to southwestern Myanmar (Dawei of Tanintharyi division), adjoining western Thailand (Sangkhla Buri district in Kanchanaburi province), and the geographically close Andaman Islands of India. On the other hand, Rohanixalus sp. 1 and Rohanixalus sp. 2 potentially represent unidentified or undescribed endemic taxa restricted to relatively higher elevations. This observation may also be considered significant in the light of remarks made by Thorell (1887), although for arachnids, noting that the fauna of Upper Iravaddi has a perfectly tropical aspect as that of Lower Iravaddi and Asia from the south in general; whereas the fauna of the Kachin mountains has a very different character. Hence, based on the currently available molecular, morphological, and geographical information, we consider the identity of R. vittatus to be as shown phylogenetically in Figures 1 View FIGURE 1 and 2 View FIGURE 2 . New topotypic collections from Bhamo can ascertain the present conclusion.
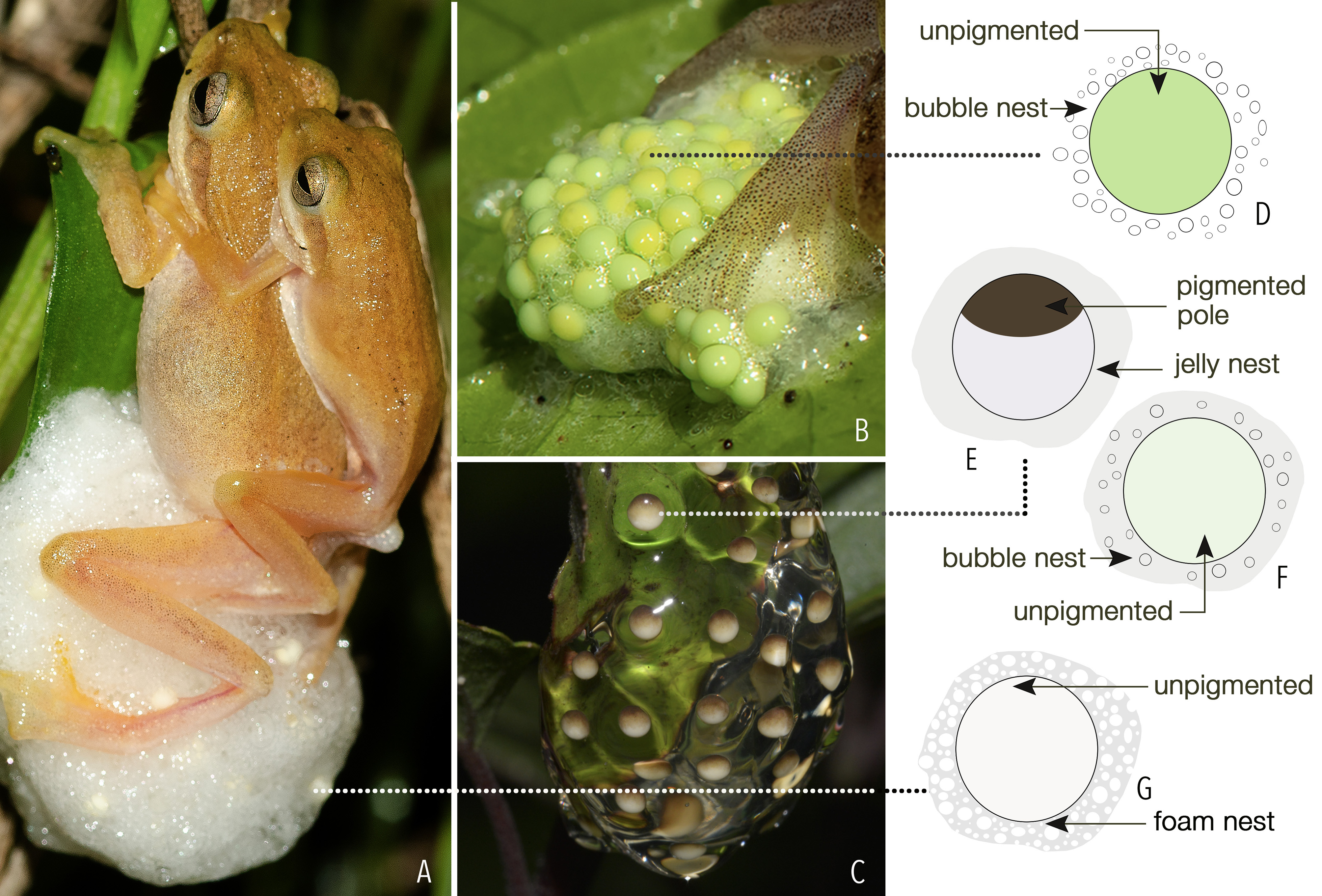
Diagnosis. Small to medium-sized adults (male SVL 21–26 mm, female SVL 24–29 mm) with slender body; snout sub-elliptical to nearly pointed in dorsal view; tympanum externally obscure; dorsum shagreened to sparsely granular; the entire dorsum, lateral surfaces (including tympanic region), and dorsal surfaces of limbs packed with fine dark brown speckles, some speckles clumping together to form irregular dark spots or streaks over the dorsum; limbs with lesser dense spots, thigh and tibia with irregularly scattered spots; a pair of prominent contrasting light coloured dorsolateral stripes starting from the tip of the snout, extending over the upper eyelid margins, and ending close to the vent on either side; a rudiment of web present between fingers III and IV at the base, webbing absent between fingers I, II, and III; foot webbing moderate, up to the second subarticular tubercle on either side of toe IV; ventral surfaces brownish or greyish white; eggs light green in colour and laid in bubble nests.
Genetic divergence. For the mitochondrial 16S gene, Rohanixalus vittatus differs from other genetically known congeners ( Fig. 1 View FIGURE 1 ) by: 9.9–11.8% from R. baladika ; 9.6–14.0% from R. hansenae ; 9.0–10.1% from R. senapatiensis ; and 8.6–10.1% from R. shyamrupus . For detailed intra-generic comparison see Table 3 View TABLE 3 .
Distribution. Prior to this study, Rohanixalus vittatus was believed to be one of the most widely distributed species in South and Southeast Asia, and reported from India, Bangladesh, Myanmar, Thailand, Laos, Cambodia, Vietnam, up to southern China. In India, it was previously reported from the northeast states of Mizoram and Nagaland (e.g., Dutta 1997; Deuti & Dutta 2002; Ao et al. 2003), excluding an earlier record from Assam that was clarified as belonging to Nagaland ( Dutta 1997). However, based on sampling in the present study, the previously reported populations of ‘ Feihyla ’ from regions across Northeast India represent Rohanixalus senapatiensis or R. shyamrupus ( Fig. 2 View FIGURE 2 ; Table 1 View TABLE 1 ). Hence, the presence of R. vittatus in mainland India, and possibly Bangladesh, is currently doubted and will require further studies or additional collections. Several other populations previously identified as ‘ vittatus ’ from regions across South and Southeast Asia, including several examined populations from China ( Yunnan Province, Guangxi Province, and Tibet), are likely to represent other Rohanixalus species. Based on our finding, we restrict the current distribution of Rohanixalus vittatus to low elevations ranging from sea level up to 200 m asl in Myanmar and Thailand, with a new record from the Andaman Islands (Middle and North Andamans) of India ( Fig. 8 View FIGURE 8 ).
Natural history and reproductive behaviour. The reproductive behaviour of Rohanixalus vittatus , including egg-laying, nesting ecology, and clutch morphology, were studied by GG, SDB, and SG under natural conditions in June 2019. The observations reported below are based on roughly 200 individuals in a population from Rangat, Middle Andamans, India .
Day 1. Location: fringe vegetation in a small-sized rice paddy; weather condition: rained for about two hours between 16:30–18:30 h.
Over 100 individuals were observed on the leaves of shrubs and herbs, including banana plants, from 0.1 to 2 m above the water level on the fringes of a small paddy field of about 15 x 20 m. Seven mating pairs were observed in axillary amplexus on the leaves of a single plant, aggressively advertising males were also observed around females. A single gravid female was observed further. The skin over the female’s belly was translucent, making the internal organs and mature ova visible through the ventral, dorsal, and lateral skin ( Fig. 8 View FIGURE 8 ), as also observed in females of R. hansenae ( Poo & Bickford 2013) . The female was perched on a shrub about 0.3 m above the water level on the edge of a waterlogged paddy field. At least six aggressively calling males were observed within a radius of about 0.5 m from the female. The female approached a calling male, which then mounted the female in axillary amplexus. The amplected pair moved to a nearby suitable oviposition site, positioned itself and remained still for up to two minutes. Then the female secreted a drop of mucous, followed by two eggs. The complete process of egg-laying was completed within 15 minutes, starting from the release of the first egg until the unmounting of the male from amplexus. The female continued to sit over the eggs and release gelatinous secretion, with which she glazed the entire egg clutch multiple times using both the hind limbs. The female remained on the egg clutch for nearly three hours without much movement. The male usually remained in close proximity to the female, without any direct contact, during most of the process ( Fig. 9 View FIGURE 9 ).
The freshly laid egg clutch was ovoid in shape (L 19 mm x B 16 mm x H 10 mm) and comprised of a sticky colourless jelly matrix containing bubbles. The clutch consisted of 120 light green eggs, unpigmented on poles. Due to the gelatinous and bubbly nature of the nest matrix, it was not possible to distinguish between or retain the individual jelly layer of each egg. Hence, each ovum was measured excluding the jelly layer, 2.0 ± 0.2 mm ( n = 30) ( Fig. 10 View FIGURE 10 ).
Day 2. The specific egg clutch was observed on the next day, during the daytime at 09:00 h (air temperature 25°C). A female was found about 30 cm away, but not on the egg clutch. At around 18:00 h, the same female visited the egg clutch and positioned herself on top of the egg mass providing cover. At around 19:00 h, the female again secreted a viscous fluid from the cloaca and glazed the substance over the egg clutch by extending her hindlimbs in a circular movement ( Fig. 11 View FIGURE 11 ).
Days 3-4. The egg clutch was observed over the subsequent two days. The same female was found sitting on the egg clutch, but only during the nighttime, and remained in that position without much movement for long durations or throughout the night. However, the gelatinous secretion was noted to be released only until the third day. On day 4, the light green coloured ova turned light grey and the jelly matrix became translucent ( Figs. 10 View FIGURE 10 , 12 View FIGURE 12 ). Further study of the same egg clutch was abandoned.
Based on additional observations from multiple egg clutches and maternal egg-attending events in the field, the following behavioural patterns were noted: (1) territorial behaviour or competition in males: seven male-male combat events involving pushing, kicking, and dislodging from females were witnessed within roughly four hours of observation. The fight bouts between males lasted from ~5–20 s. Out of the seven combat events, five involved multiple males ( Fig. 12 View FIGURE 12 ); (2) multiple males mating with a single female: immediately after mounting of a male with female and initiation of the egg-laying process, two additional males were observed to mount on either side of the amplected pair ( Fig. 12 View FIGURE 12 ); (3) egg-attendance possibly only during the late evening and nighttime, but individuals can be occasionally observed in nearby vegetation during the day time; (4) attendance of a single egg-clutch by multiple females ( Fig. 11 View FIGURE 11 ); (5) maternal attendance of the developing egg-clutch at all stages until hatching ( Fig. 11 View FIGURE 11 ); (6) assisted release of hatchlings and young tadpoles from the bubble nest by the female upon disturbance: when developed embryos are disturbed in the presence of a female, she rapidly and repeatedly kicks the egg clutch with her hind feet, consequently dismantling the smooth clutch surface, dislodging the hatchlings and young tadpoles from the jelly matrix, and propelling them to the water below; (7) laying of fresh eggs over existing clutches or in their close proximity ( Fig. 11 View FIGURE 11 ).
Vocalisation. The calls of a Rohanixalus vittatus male ( SDBDU 2019.4032 ) were recorded at Rangat, Middle Andamans, India on 13 June 2019, by GG, SG, and SDB. The ambient temperature at the time of recording was 30°C (dry bulb) and 28.5°C (wet bulb). The males were observed to produce a single type of call with pulsatile temporal structure. Calls were not delivered in groups and had uniform intervals. A typical male advertisement call has a duration of 14.2 ms; a short rise time of about 0.5 ms and fall time of 13.6 ms; and six pulses delivered at a rate of 472.5 pulses/second. The spectrum is characterized by a single broad peak with mean dominant frequency of 4.9 kHz ( Fig. 9 View FIGURE 9 ) .
We compared the call of R. vittatus with that of R. shyamrupus (SDBDU 4508 recorded at Nagaland, India, by a Systematic Lab team), a closely related member of the genus. While the overall call structure was observed to be similar in both species, the call of R. vittatus differed from that of R. shyamrupus primarily by faster pulse rate, 472.5 pulses/second (vs. slower, 173.4 pulses/second) and higher overall dominant frequency, 4.9 kHz (vs. lower, 3.9 kHz).
Based on available literature, we also compared R. vittatus and R. hansenae calls reported from two phylogenetically distinct populations in Thailand (‘ Feihyla ’ hansenae Groups I and II in Aowphol et al. 2013 ). Although the comparison was limited due to methodological differences, especially with respect to acoustic terminologies, the overall call structure in the two species was similar. However, the call of R. vittatus differed by relatively shorter call duration, 21.2 ms (vs. longer, 26.89 ms in ‘ Feihyla ’ hansenae group I and 99.94 ms in ‘ F. ’ hansenae group II), and lower dominant frequency of 4.9 kHz (vs. higher, 5.1–5.2 kHz for ‘ F. ’ hansenae groups I and II ).
Our recording of R. vittatus from Andamans could also not be reliably compared with that of the R. vittatus population from Thailand (‘ Feihyla vittata ’ group II in Aowphol et al. 2013). Although the two were similar in overall structure, they differed in duration (21.2 ms in Andamans population vs. 40.25 ms in Thailand population) and the overall dominant frequency (4.9 kHz in Andamans population vs. 3.9 kHz in Thailand population). Further acoustic studies are required for a proper understanding of intra- and interspecific variations among the calls of species in the genus Rohanixalus .
Tadpole morphology. The larval description for Rohanixalus vittatus is based on Gosner stage 35 tadpole ( SDBDU 2019.4052 ) collected from a freshly laid egg clutch from Rangat, Middle Andamans, India on 15 June 2019, by GG, SDB, and SG .
External morphology. Small-sized tadpole (tl 24.75, svl 8.10, su 6.31); body oval-shaped in dorsal view (bh 3.68, bw 4.25); eyes rounded, positioned dorsolaterally (pp 3.95), eye diameter (ed 1.63) 38% of the body width (bw 4.25); snout shape rounded in dorsal and lateral view (from eyes to the base of upper labium); umbraculum absent; nasolacrimal duct absent; narial depressions located closer to the snout than the eyes (rn 1.09, np 1.95, nn 1.89); narial depressions and surrounding pigmentation not clearly visible in dorsal view; spiracle present as a muscular flap on the left side of the body, opens mid-laterally (ss 5.65); a medial vent tube present, tubular in structure, and with the aperture opening dextrally compared to the plane of the ventral fin; tail (vt 16.65, ht 4.62) composed of bilateral myotomic muscle masses divided by V-shaped septa, with unequal tail membranes on either side of the tail musculature; dorsal fin originates anterior to the tail body junction, whereas the ventral fin originates near the vent tube; margin of the lower fin not parallel to the margin of tail muscle; tail musculature strong, extends up to the end of the tail tip with a narrow terminal part; tail-muscle height (tmh 2.22) 60% of the body height (bh 3.68) and tailmuscle width (tmw 1.82) 43% of body width (bw 4.25) ( Fig. 13 View FIGURE 13 ).
Mouth (oral disc): Oral disc elliptical in shape, anteroventrally positioned, moderately small, oral disc width (odw 1.91) 45% of body width and 24% of snout to vent length; mouth consisting of lower and upper labia, with the lower being larger; lateral emarginations present on both sides, bordered by continuous small blunt marginal papillae; upper labium with a single row of small and blunt marginal papillae, marginal papillae not continuous throughout the margin and contain a medial gap in between; lower labium with a row of elongated and blunt marginal papillae, and a second row of blunt submarginal papillae, a ventral gap present in both the rows of papillae; two upper tooth ridges and three lower ridges are present; uniserial tooth rows present for each tooth ridge, composed of pointed and uniformly sized labial teeth except for some inconsiderable occasions (like the lateral ends where the teeth tend to become smaller in size); A2 have natural gaps between them while A1 shows gaps made by teeth degeneration; P1 indicates a gap comparatively smaller to A2; labial tooth row formula: 2(2)/3(1); keratinized upper and lower jaw sheaths, with an inverted, smooth, U-shaped upper beak and a V-shaped lower beak, both mostly black with pale bases and serrated margins.
Development of mouth parts: The number of tooth rows and presence of papillae differs as development of the tadpole progresses; upper labium possesses only marginal papillae throughout its various developmental stages, whereas the lower labium possesses rows of marginal and submarginal papillae that increase in number and size as the larvae develop; the row of submarginal papillae is not continuous. Tooth rows are developed in the early tadpole stages (Gosner 25); A1 have no medial gap till stage 30 but have a wide medial gap by stage 35, after which the teeth start degenerating; A2 have a wide medial gap from Gosner 25 and start degenerating at Gosner 35; P1 indicates a narrow gap starting from Gosner 25; the oral disc is well-developed at stage 30, with two anterior (A1–A2) and three posterior tooth rows (P1-P3); initially anterior tooth rows degenerate; A1 and A2 degenerate at Gosner 35; P1–P3 remain intact even in Gosner 35.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Rhacophorinae |
|
Genus |
Rohanixalus vittatus ( Boulenger, 1887 )
| Biju, S. D., Garg, Sonali, Gokulakrishnan, G., Chandrakasan, Sivaperuman, Thammachoti, Panupong, Ren, Jinlong, Gopika, C., Bisht, Karan, Hamidy, Amir & Shouche, Yogesh 2020 |
Rohanixalus vittatus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
R. vittatus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
R. vittatus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus cf. shyamrupus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
R. shyamrupus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus vittatus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
R. vittatus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
Rohanixalus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |
R. vittatus
| Biju & Garg & Gokulakrishnan & Chandrakasan & Thammachoti & Ren & Gopika & Bisht & Hamidy & Shouche 2020 |