Cnemaspis peninsularis, Grismer & Wood & Anuar & Riyanto & Ahmad & Muin & Sumontha & Grismer & Onn & Quah & Pauwels, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3880.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:03A6448A-25D7-46AF-B8C6-CB150265D73D |
|
DOI |
https://doi.org/10.5281/zenodo.5708558 |
|
persistent identifier |
https://treatment.plazi.org/id/03FA0350-FFDA-2575-FF51-CCCAFDB92F66 |
|
treatment provided by |
Felipe |
|
scientific name |
Cnemaspis peninsularis |
| status |
sp. nov. |
Cnemaspis peninsularis View in CoL sp. nov.
Peninsular Rock Gecko
Figs. 60 View FIGURE 60 , 61 View FIGURE 61
Gonatodes kendalli Flower 1896:833 (in part), 1899:627 (in part); Ridley, 1899:193; Boulenger 1912:38 (in part); Sworder 1925:63; Smith 1930:16 (in part); Nicholls 1949:48
Gonatodes kendallii Smith 1925:23 (in part)
Cnemaspis kendalli Henrickson, 1966:55 ; Bullock 1966:94; Dring, 1979:220; Denzer & Manthey, 1991:313; Lim & Lim, 1992:122
Cnemaspis kendallii Manthey & Grossmann 1997:212 View in CoL (in part); Das & Bauer 1998:12 (in part); Grandison, 1972:80; Werner & Chou, 2002:185; Das & Grismer, 2003:549; Grismer & Das 2006:5 (in part); Grismer, Youmans, Wood & Grismer, 2006:112; Grismer & Ngo 2007:486 (in part); Baker & Lim, 2008:78; Chan & Grismer 2008:55 (in part); Grismer 2008:30; Grismer & Chan 2008:5 (in part); Grismer, Chan, Nurolhuda & Sumontha 2008a:57 (in part); Grismer, Grismer, Wood & Chan 2008b:24 (in part); Grismer & Chan 2009:30 (in part); Grismer, Norhayati, Chan, Belabut, Muin, Wood, & Grismer 2009:59 (in part); J. Grismer, Grismer & Thou 2010:30 (in part); Grismer, 2010:59 (in part); Grismer & Chan 2010:61 (in part); Grismer, Chan, Quah, Muin, Savage, Grismer, Norhayati, Greer, Remegio 2010c:64 (in part); Grismer, Ngo & Grismer 2010b:57 (in part); Grismer, Sumontha, Cota, Grismer, Wood, Pauwels & Kunya 2010a:12 (in part); Grismer 2011a:330 (in part), 2011b:112 (in part); Wood, Quah, Shahrul, & Muin 2013:546 (in part); Grismer, Wood, Amirrundin, Sumarli, Vazquez, Chan, Ismail, Nance, Muhammad, Mohamad, Syed, Kuss, Murdoch & Cobos 2014:449.
Holotype. Adult female LSUHC 8965 View Materials collected on 7 June 2008 by L. L. Grismer, P. L. Wood, Jr., J. L. Grismer and Chan K. O. at 1030 hrs from the base of Gunung Ledang Johor, Peninsular Malaysia (02°20.25’ N, 102°37.11’ E) at 100 m in elevation. GoogleMaps
Paratypes. Adult male LSUHC 8966 View Materials has the same data as the holotype. GoogleMaps Adult male LSUHC 4756 View Materials collected on 23 July 2002 by J. L. Grismer at the waterfall on Pulau Tinggi, Johor, Peninsular Malaysia (02°18.013’ N, 104°06.261 E) at 210 m in elevation. GoogleMaps Adult male LSUHC 5731 View Materials collected on 30 August 2003 by T. A. Youmans on Pulau Babi Besar, Johor, Peninsular Malaysia (02°26.166 N, 103°58.466 E) at 55 m in elevation. GoogleMaps Adult male LSUHC 6213 View Materials collected on 30 June 2004 by L. Lee Grismer on the Tekek-Juara Trail , Pulau Tioman , Pahang, Peninsular Malaysia (02°48.433 N, 104°9.525 E) at 260 m in elevation. GoogleMaps Adult female LSUHC 8210 View Materials and adult male LSUHC 8126 View Materials collected on 4 September 2006 by L. L. Grismer at Selai , Lubuk Tapah , Endau-Rompin , Johor, Peninsular Malaysia (02°25.129’ N, 103°15.409’ E) at 102 m in elevation. GoogleMaps Adult male LSUHC 9376 View Materials collected on 8 September 2009 by Chan, K. O. and L. Lee Grismer on Pulau Tenggol , Terengganu, Peninsular Malaysia (04°48.111 N, 103°40.478’ E) at 83 m in elevation. GoogleMaps Adult males ( LSUHC 10710–11 View Materials ) collected on 24 June 2008 by Chan Kin Onn at 1030 hrs from Bukit Hangus , Pahang, Peninsular Malaysia (04°16.142’ N, 102°13.370’ E) at 10 m in elevation. GoogleMaps Adult male LSUHC 10454 View Materials collected on 2 June 2011 by Evan S. H. Quah from the Nee Soon Swamp, Singapore (01°48.40 N, 103°49.41 E) at 20 m in elevation GoogleMaps .
Diagnosis. Maximum SVL 60.0 mm; 10 or 11 supralabials; 7–10 infralabials; keeled ventrals; no precloacal pores; moderately prominent dorsal tubercles; 17–25 paravertebral tubercles; dorsal body tubercles generally randomly arranged; tubercles absent to weak on flanks; caudal tubercles encircling tail; no tubercles in lateral caudal furrows; ventrolateral and lateral rows of caudal tubercles present; subcaudals keeled; no single, median row of enlarged, subcaudal scales; one or two postcloacal tubercles on either side of base of tail; no enlarged femoral, subtibial, or submetatarsal scales; subtibials keeled; 27–33 subdigital lamellae on fourth toe; regenerated tail yellow in males; posterior portion of original tail in males black (Tables 6,7).
Description of holotype. Gravid female; SVL 55.2 mm; head oblong in dorsal profile, moderate in size (HL/ SVL 0.27), somewhat narrow (HW/SVL 0.16), flattened (HD/HL 0.37), distinct from neck; snout short (ES/HL 0.47), slightly concave in lateral profile; postnasal region constricted medially, flat; scales of rostrum weakly keeled, slightly raised, same size as similarly shaped scales on occiput; low, supraorbital ridges; very weak frontorostral sulcus; canthus rostralis not very discernable; eye large (ED/HL 0.19); extra-brillar fringe scales largest anteriorly; pupil round; ear opening oval, taller than wide; rostral concave dorsally, dorsal 75% divided by longitudinal groove; rostral bordered posteriorly by two large supranasals and external nares, and laterally by first supralabials; 10R,L raised supralabials decreasing in size posteriorly; 8R,9L infralabials, decreasing in size slightly posteriorly; nostrils elliptical, oriented dorsoposteriorly; bordered posteriorly by small, granular, postnasal scales; mental large, triangular, concave medially, bordered posteriorly by two large, rectangular, lateral postmentals of similar size and one smaller azygous scale; gular scales raised, smooth; throat scales larger, raised, weakly keeled.
Body slender, elongate (AG/SVL 0.48); small, keeled, dorsal scales generally equal in size throughout body, intermixed with larger, multicarinate tubercles more or less randomly arranged; tubercles extend from occiput to base of tail; tubercles absent from lower flanks, moderate in size; 22 paravertebral tubercles; pectoral and abdominal scales raised, keeled, not elongate, same size throughout; abdominal scales slightly larger than dorsals; no precloacal pores; forelimbs moderately long, slender (FL/SVL 0.19); dorsal scales of brachium raised, keeled; dorsal scales of forearm raised, keeled; ventral scales of brachium smooth, raised, juxtaposed; ventral scales of forearm weakly keeled, raised, juxtaposed; palmar scales smooth, juxtaposed, raised; digits long with an inflected joint; claws recurved; subdigital lamellae unnotched; lamellae wide throughout digit; interdigital webbing absent; fingers increase in length from first to fourth with fourth longer than fifth; hind limbs slightly longer and thicker than forelimbs (TBL/SVL 0.22); dorsal scales of thigh keeled, raised, juxtaposed; scales of anterior margin of thigh keeled; ventral scales of thigh keeled; subtibial scales raised, keeled, juxtaposed, with no enlarged anterior row; plantar scales smooth, juxtaposed, raised; no enlarged submetatarsal scales beneath first metatarsal; digits elongate with an inflected joint; claws recurved; subdigital lamellae unnotched; lamellae beneath first phalanges granular proximally but wider distally throughout digit; interdigital webbing absent to weak; toes increase in length from first to fourth with fourth being slightly longer than fifth; 31 subdigital lamellae on fourth toe; caudal scales arranged in segmented whorls; dorsal caudal scales flat anteriorly, keeled, juxtaposed; deep middorsal and lateral caudal furrows; subcaudal scales keeled; no median row of enlarged keeled subcaudal scales; caudal tubercles encircle tail; tubercles absent from lateral furrows.
Color pattern in life. Dorsal ground color yellowish; head and body overlain with irregularly shaped pattern of interconnected brownish markings highlighting white to yellowish irregularly shaped blotches; small, black, elongate medial marking on nape followed by similarly colored, paired, elongate, paravertebral markings extending to midway down body; similar vertebral markings extend from midbody to base of tail transforming into poorly defined, diffuse, dark caudal bands; limbs bearing a faint, brownish banding pattern becoming more evident distally; digits banded; ventral ground color beige; faint, darker reticulum on belly; posterior subcaudal region dark.
Variation. The type series shows a modest array of color pattern variation that is not necessarily related to substrate matching although this species does tend to show differences in overall hue with respect to substrate (see Geographic variation below). LSUHC 5731 View Materials and 6253 match the holotype in general coloration and pattern although both lack the larger dark markings and appear less mottled overall. Both differ from the holotype in having a dark belly with distinct white spots as do LSUHC 10454 View Materials and 10710–11. The other specimens show a modest degree of belly mottling. LSUHC 10710–11 View Materials have a much darker color pattern that highlights the overlying lighter markings as well as a distinct, thin, whitish, nuchal loop. A distinct nuchal loop also occurs in LSUHC 4756 View Materials , 8210 View Materials , 8966 View Materials , and 10454. LSUHC 9376 View Materials has a faded nearly unicolor dorsal pattern bearing only faint dark and light spots and LSUHC 8126 View Materials has a unicolor regenerated tail. Sexual dimorphism in caudal coloration is discussed below (see Comparisons). Meristic and mensural variation is listed in Table 14 View TABLE 14 and various color patterns can be seen in Figures 60 View FIGURE 60 and 61 View FIGURE 61 and in Grismer (2011a) .
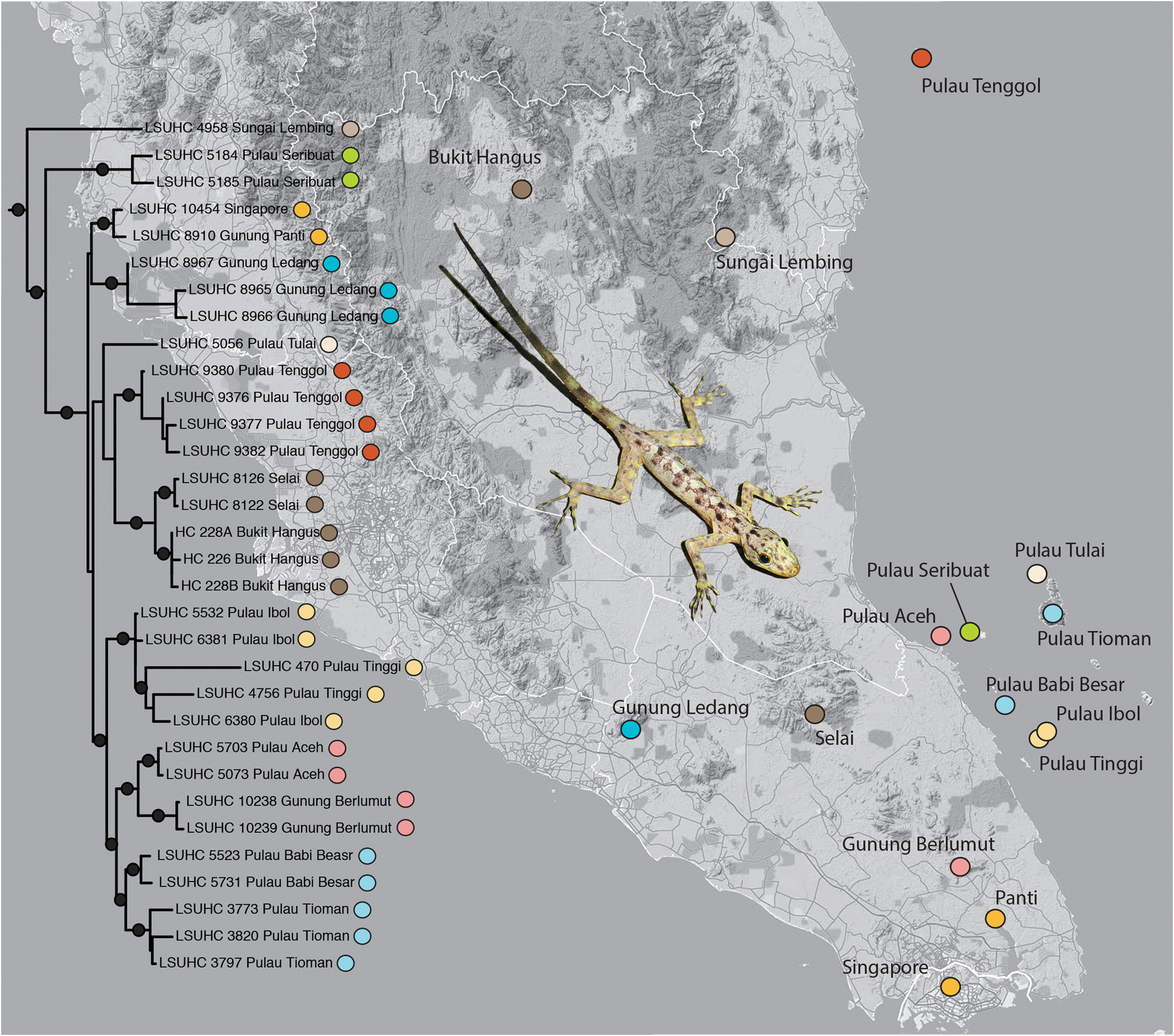
Distribution. Cnemaspis peninsularis sp. nov. ranges as far north as Bukit Hangus, Pahang on the peninsula and to Pulau Tenggol off the east coast, and southward to Singapore ( Grismer 2011a: Fig. 4 View FIGURE 4 ). In the Seribuat Archipelago, Grismer et al. (2006) reported C. peninsularis sp. nov. from the islands of Aceh, Babi Besar, Babi Hujung, Ibol, Sembilang, Seribuat, Sibu, Sibu Tenggah, Tinggi, Tioman, and Tulai ( Fig. 4 View FIGURE 4 ).
Natural History. According to Grismer (2011a) and references therein, Cnemaspis peninsularis sp. nov. is a scansorial, diurnal gecko found on logs, tree trunks, low vegetation, and rocks ( Figs. 60 View FIGURE 60 , 61 View FIGURE 61 ). It is a common inhabitant of both primary and secondary, lowland and hill, dipterocarp forests and to a lesser extent peatswamps and ranges up to approximately 500 m in elevation. During the day, C. peninsularis sp. nov. is active on trees and rocks beneath closed canopy forests and does not restrict its movements to dark, shaded surfaces as is commonly seen in many other species of Cnemaspis . On Pulau Tioman, Pahang, Grismer (2011a) noted that lizards may bask in sun spots on the trunks of trees. Werner and Chou (2002) indicated C. peninsularis sp. nov. is a sit and wait predator that usually perches head down on tree trunks waiting to ambush prey. When threatened, males often curl their tail over their back to display the yellow underside ( Fig. 61 View FIGURE 61 ). This display is usually exaggerated by slowly moving the tail from side to side and performed just prior to fleeing to take refuge within a rock crack or beneath exfoliating bark. At night, the ground color of C. peninsularis sp. nov. becomes nearly white and somewhat transparent, highlighting the dark dorsal spots on the body and the black tail in the males ( Figs. 60 View FIGURE 60 , 61 View FIGURE 61 ). During this time, lizards are commonly seen sleeping on tree trunks, leaves, rocks, and clinging to the underside of leaves as high as 10 m above the ground. Grismer (2011a) reported gravid females on several islands in the Seribuat Archipelago from March through August; during June at Gunung Ledang, Johor; and at Endau-Rompin, Johor during September. In Singapore, gravid females have been observed during August and pairs of eggs stuck to rocks and cement structures have been found during November and December ( Werner & Chou 2002). Hatchlings and gravid females in Singapore have been observed during December ( Werner & Chou 2002). These data suggest C. peninsularis sp. nov. breeds year round. Bullock (1966) reported finding ants, beetles, earthworms, millipedes, and soil in the stomachs of lizards from Pulau Tioman indicating that foraging takes place on the ground.
Etymology. The specific epithet peninsularis is an adjective in reference to the distribution of this species being restricted to Peninsular Malaysia and Singapore and their adjacent islands.
Geographic variation. Geographic variation in Cnemaspis peninsularis sp. nov. does not show geographically related trends as seen in some other species of lizards from Peninsular Malaysia ( Grismer 2011a) but there is some noteworthy localized variation in some populations. Lizards from Pulau Tioman , Pahang generally have a more boldly marked abdomen than lizards from populations of Peninsular Malaysia. This is especially true in adult males. In extreme cases, the bellies of some males may be dark brown with white spots and that pattern may extend onto the undersides of the hind limbs. The dorsal pattern of lizards from Pulau Ibol , Johor is nearly unicolor brown. A similar pattern occurs in lizards from Sungai Lembing , Pahang except that lizards from here maintain the large, black, elongate dorsal blotches and appear superficially similar to C. baueri of Pulau Aur , Johor.
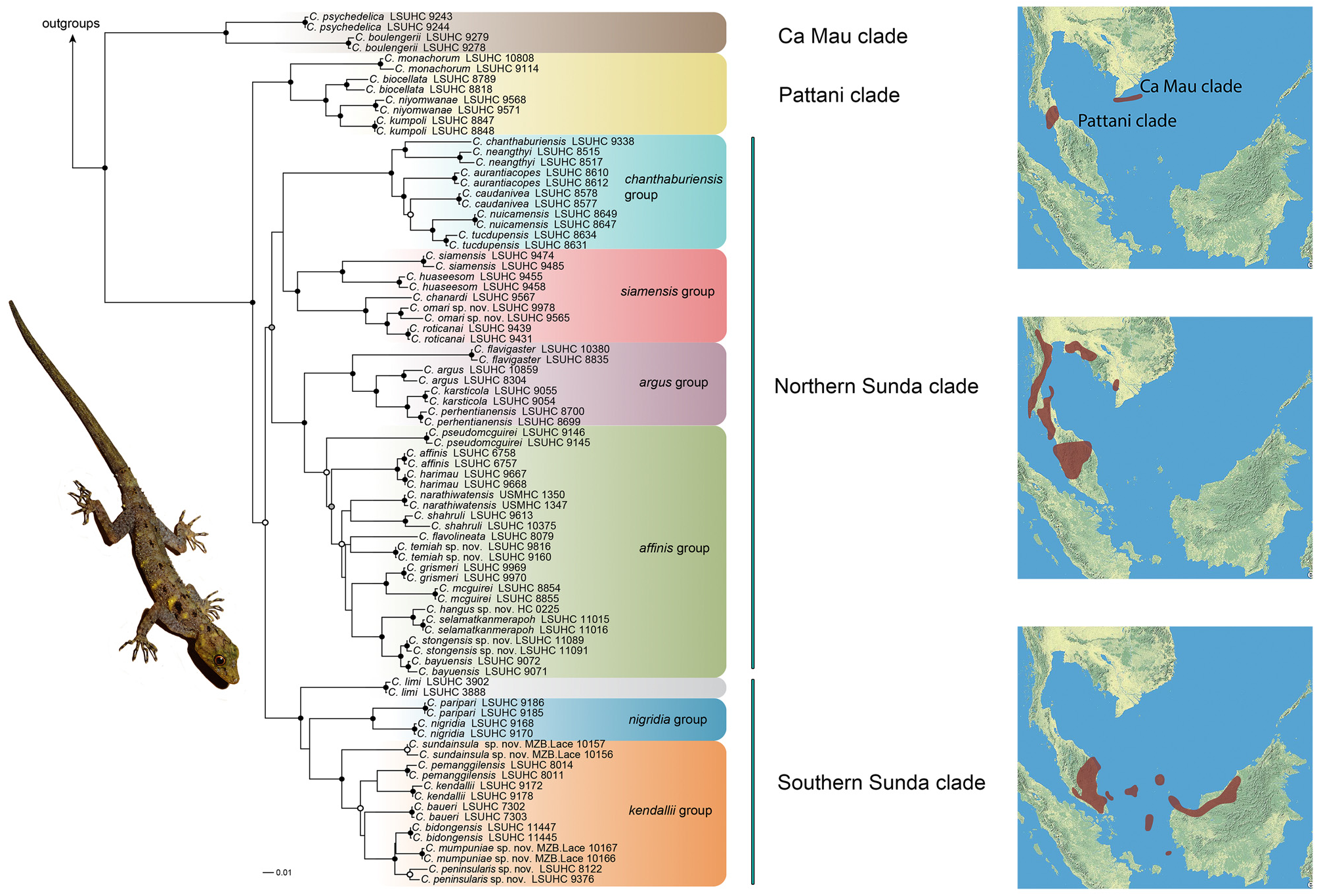
Comparisons. Cnemaspis peninsularis sp. nov. is a member of the Southern Sunda clade which includes C. limi , C. nigridia , C. paripari , C. kendallii , C. sundainsula sp. nov., C. pemanggilensis , C. baueri , C. mumpuniae sp. nov. and C. bidongensis . Within this clade, it is part of an unresolved polytomy that composes C. kendallii , C. sundainsula sp. nov., C. pemanggilensis , C. mumpuniae sp. nov., C. bidongensis , C. peninsularis sp. nov., and C. baueri of the kendallii group ( Fig. 2 View FIGURE 2 ). Cnemaspis peninsularis sp. nov. is easily separated from C. limi by being much smaller (maximum SVL 55.2 mm versus 88.2 mm); having fewer paravertebral tubercles (17–25 versus 25–35); having keeled versus smooth subcaudal scales; the presence versus the absence of a ventrolateral row of caudal tubercles; having caudal tubercles that encircle the tail versus not having tubercles that encircle the tail; and lacking versus having white caudal tubercles. From C. paripari , C. peninsularis sp. nov. lacks precloacal pores as opposed to having them; has fewer paravertebral tubercles (17–25 versus 26–31); has as opposed to lacks tubercles on the flanks; has versus lacks a ventrolateral row of caudal tubercles; has caudal tubercles that encircle the tail versus not having tubercles encircling the tail; lacks versus has an enlarged, median subcaudal scale row; and males lack as opposed to having a yellow head, limbs, and back and the posterior one-half of the original tail being white. Within the kendallii group, C. peninsularis sp. nov. is easily distinguished from C. sundainsula sp. nov., C. pemanggilensis , and C. baueri by being much smaller (maximum SVL 55.2 mm versus 67.4–84.5 mm) and from C. sundainsula sp. nov. it is further separated by having keeled versus smooth ventral scales, and caudal tubercles that encircle the tail rather than not having such tubercles. Cnemaspis peninsularis sp. nov. is further separated from C. pemanggilensis by having fewer paravertebral tubercles (17–25 versus 30–37) and lacking as opposed to having an enlarged, median row of keeled subcaudal scales. From C. baueri , C. peninsularis sp. nov is further differentiated by lacking an enlarged, median row of keeled subcaudal scales and not having a uniform brown dorsal color pattern bearing large, elongate black blotches on the nape and in the shoulder region. Cnemaspis peninsularis sp. nov. can not be differentiated from C. kendallii (with which it was previously considered conspecific) on the basis of scale counts. However, these species do differ notably in that the abdomen of C. peninsularis sp. nov. is mottled with a diffuse, dark reticulum enclosing lighter spots whereas in C. kendallii the abdomen is beige and generally immaculate; the posterior two-thirds of the original tail in adult male C. peninsularis sp. nov. is black dorsally and ventrally and in adult male C. kendallii the tail is banded dorsally throughout its length and the subcaudal region is essentially immaculate white. The regenerated tail in adult male C. peninsularis sp. nov. is yellow and immaculate dorsally and ventrally whereas that of C. kendallii is straw colored with small black flecks dorsally and the subcaudal region is white and immaculate. Additionally, C. kendallii has a row of nearly contiguous tubercles on the lateral margings of the occipital region bordering the nape which are nearly always absent in C. peninsularis sp. nov. Another row of tubercles generally absent in C. peninsularis sp. nov. occurs immediately anterior to the shoulder region in C. kendallii . All these tubercles are usually accentuated by being white. Within the kendalli group, C. peninsularis sp. nov. is most closely related to C. bidongensis and C. mumpuniae sp. nov. ( Fig. 2 View FIGURE 2 ). It differs from C. mumpuniae in lacking a brick-red ground color and a thin, white, nuchal loop. It is differentiated further from C. bidongensis sp. nov. by lacking as opposed to having an enlarged, median, subcaudal scale row.
Relationships. Cnemaspis peninsularis sp. nov. forms a polytomy with C. bidongensis from Pulau Bidong, Peninsular Malaysia and C. mumpuniae sp. nov. from Pulau Natuna Besar, Indonesia ( Fig. 2 View FIGURE 2 ).
Remarks. Cnemaspis peninsularis sp. nov. is the most widely distributed species withn a genus that is composed primarily of microhabitat specialist with highly circumscribed distributions ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ). One of the reasons for its relatively wide distribution is that it is a habitat generalist active both day and night on all rocky and vegetative substrates. To encompass the phylogeographic structure within C. peninsularis sp. nov., we sampled 32 specimens from throughout the extent of its distribution from Singapore in the south to Bukit Hangus, Pahang, and Pulau Tenggol, Terengganu in the north ( Fig. 62 View FIGURE 62 ) as well as from seven of the 11 islands on which it is known to occur in the Seribuat Archipelago ( Fig. 62 View FIGURE 62 ). An ND2 phylogeny shows that C. peninsularis sp. nov. is composed of three well-supported, major lineages ( Fig. 62 View FIGURE 62 ). The basal lineage is represented by a northern population from Sungai Lembing, Pahang and its sister lineage is composed of a southern population from Pulau Seribuat, Johor of the Seribuat Archipelago and its sister lineage containing the remaining populations that generally encompasses the entire range of C. peninsularis sp. nov. ( Fig. 62 View FIGURE 62 ). The overall phylogeographic structure within the latter widespread population is polytomous with little concordance between geographic distribution and phylogenetic substructuring ( Fig. 62 View FIGURE 62 ). We consider this lack of geographic clustering to be evidence of gene flow within this widely distributed, habitat generalist (see Grismer et al. [2012] and Johnson et al. [2012] for other gekkonid examples with similar phylogeographic structure from Peninsular Malaysia). Additionally, the phylogeny indicates that the presence of C. peninsularis sp. nov. in the Seribuat Archipelago may be due to sequential vicariant events associated with episodic changes is sea levels or independent dispersal events. The first event established the Pulau Seribuat population followed by the establishment of a population on Pulau Tulai. The widespread clade of insular populations in the Seribuat Archipelago may have resulted from the most recent vicariant event and being that the peninsular, Gunung Berlumut population is deeply embedded within this island clade, suggests an upstream recolonization of Peninsular Malaysia may have occurred ( Fig. 62 View FIGURE 62 ). These hypotheses and others are currently being tested with fine-scaled geographic sampling and re-analysis (Wood & Grismer in prep.).
The uncorrected pairwise genetic distances among the three major lineages of Cnemaspis peninsularis sp. nov. ranges from 1.1%–9.9% whereas that within the most widespread lineage (i.e. the lineage that does not include Sungai Lembing and Pulau Seribuat; Fig. 62 View FIGURE 62 ) ranges from 1.1%–6.7% ( Table 5 View TABLE 5 ). The distances between the Sungai Lembing and Pulau Seribuat populations to each other and all other C. peninsularis sp. nov. are commensurate with that between other species of gekkonids (see Grismer et al. 2013b) and their specific status will also be evaluated (Wood & Grismer in prep.).
Additional material examined. Peninsular Malaysia: Johor: Bunker Trail ZRC 2.5602; Endau-Rompin LSUHC 7691, 8122, 8126, 8191, 8210; Gunung Ledang ZRC 2.5437–38, LSUHC 8965–67; Pulau Babi Besar LSUHC 5731–34; Pulau Babi Hujung LSUHC 5749–52; Pulau Ibol LSUHC 6380–83; Pulau Sembilang LSUHC 5244; Pulau Seribuat LSUHC 5184–87, 5198, 5211; Pulau Tinggi LSUHC 4707, 4756–57, 4765–67; Pulau Tulai LSUHC 3894, 5056–58. Pahang: Bukit Ringgit DWNP 2231; Gemas ZRC 2.1105–06; Jerantut ZRC 2.1101; Kuala Gandah DWNP 475–76; Lakum Forest Reserve DWNP 2278–79; Pulau Tioman DWNP 1833, LSUHC 3773–75, 3797, 3811, 3820, 3841, 3878–88, 4566, 4570, 4615, 4658–59, 4666; 5436, 5445–46, 5454, 5462, 5477, 5482, 6213–18, 6224, 8036; Sungai Lembing LSUHC 4954, 4958. Negeri Sembilan: Gallah Forest Reserve DWNP 2281. Selangor: Sungai Lalang DWNP 169; Ulu Gombak DWNP 1828. Singapore: ZRC 2.107–08, 2.3014, 2.3520, 2.3544, 2.4992, 2.5644, 2.5891.
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cnemaspis peninsularis
| Grismer, Lee, Wood, Perry L., Anuar, Shahrul, Riyanto, Awal, Ahmad, Norhayati, Muin, Mohd A., Sumontha, Montri, Grismer, Jesse L., Onn, Chan Kin, Quah, Evan S. H. & Pauwels, Olivier S. A. 2014 |
Cnemaspis kendallii
| Grismer, L. L. & Grismer, J. L. & Wood, P. L. Jr. & Ngo, V. T. & Neang, T. & Chan, K. O. 2011: 330 |
| Grismer, J. L. & Grismer, L. L. & Thou, C. 2010: 30 |
| Grismer, J. L. & Grismer, L. L. & Thou, C. 2010: 59 |
| Grismer, J. L. & Grismer, L. L. & Thou, C. 2010: 61 |
| Grismer, L. L. & Chan, K. O. & E. Quah & Mohd, A. M. & A. E & Savage, J. L. & Grismer & Norhayati, A. & Greer III & Remegio, A. - C. 2010: 64 |
| Grismer, L. L. & Ngo, V. T. & Grismer, J. L. 2010: 57 |
| Grismer, L. L. & Sumontha, M. & Cota, M. & Grismer, J. L. & Wood, P. L. Jr. & Pauwels, O. S. G. & Kunya, K. 2010: 12 |
| Grismer, L. L. & Chan, K. O. 2009: 30 |
| Baker, N. & Lim, K. 2008: 78 |
| Grismer, L. L. & Grismer, J. L. & Wood, Jr., P. L. & Chan, K. O. 2008: 55 |
| Grismer, L. L. & Grismer, J. L. & Wood, Jr., P. L. & Chan, K. O. 2008: 30 |
| Grismer, L. L. & Grismer, J. L. & Wood, Jr., P. L. & Chan, K. O. 2008: 5 |
| Grismer, L. L. & Chan, K. O. & Nurolhuda, N. & Sumontha, M. 2008: 57 |
| Grismer, L. L. & Grismer, J. L. & Wood, Jr., P. L. & Chan, K. O. 2008: 24 |
| Grismer, L. L. & Ngo, V. T. 2007: 486 |
| Grismer, L. L. & Das, I. 2006: 5 |
| Grismer, L. L. & Youmans, T. M. & Wood, P. L. Jr. & Grismer, J. L. 2006: 112 |
| Das, I. & Grismer, L. L. 2003: 549 |
| Werner, Y. L. & Chou, L. M. 2002: 185 |
| Bauer, A. M. & Das, I. 1998: 12 |
| Manthey, U. & Grossmann, W. 1997: 212 |
| Grandison, A. G. C. 1972: 80 |
Cnemaspis kendalli
| Lim, K. K. P. & Lim, F. L. K. 1992: 122 |
| Denzer, W. & Manthey, U. 1991: 313 |
| Dring, J. C. 1979: 220 |
| Bullock, J. A. 1966: 94 |
Gonatodes kendallii
| Smith, M. A. 1925: 23 |
Gonatodes kendalli
| Nicholls, L. 1949: 48 |
| Smith, M. A. 1930: 16 |
| Sworder, G. H. 1925: 63 |
| Boulenger, G. A. 1912: 38 |
| Ridley, H. N. 1899: 193 |
| Flower, S. S. 1896: 833 |