Carientothrips Moulton, 1944
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3821.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:C93F0714-35E6-46BE-8754-D5B17C4F7FF5 |
|
DOI |
https://doi.org/10.5281/zenodo.5117644 |
|
persistent identifier |
https://treatment.plazi.org/id/03FA87CC-FF9A-2362-FF5B-F8ADFDE36B04 |
|
treatment provided by |
Felipe |
|
scientific name |
Carientothrips Moulton |
| status |
|
Carientothrips Moulton View in CoL
Bolothrips (Carientothrips) Moulton, 1944: 306 . Type species Bolothrips (Carientothrips) fijiensis Moulton , by monotypy. This genus is no longer considered to be closely related to Bolothrips , a genus that is now placed in the subtribe Compsothripina in which the species have three (rarely two) sensoria on the fourth antennal segment. Mound (1974b) provided a key to the 18 species of Carientothrips then recognised. However, with the six new species described here ( alienatus , calami , horni , palumai , snowi , tasmanica ), a further species recalled from synonymy ( flavitibia ), and two species transferred to Nesothrips ( badius, capricornis ), the total is now 23, of which 18 are from Australia and with one each from Japan (japonicus), New Guinea ( grayi ), Fiji ( fijiensis ), Rapa (Austral Islands) (biformis), and the extreme south of South America (Tierra del Fuego and Falkland Islands) ( denticulatus ). Judging from the gut contents, all of the Australian species feed on whole fungal spores. Many of them breed on dead leaves, particularly of Eucalyptus trees, one is specific to dead rattan palm fronds ( Calamus ), but others are found breeding at the base of grasses, rather like Bolothrips species in the Holarctic Region.
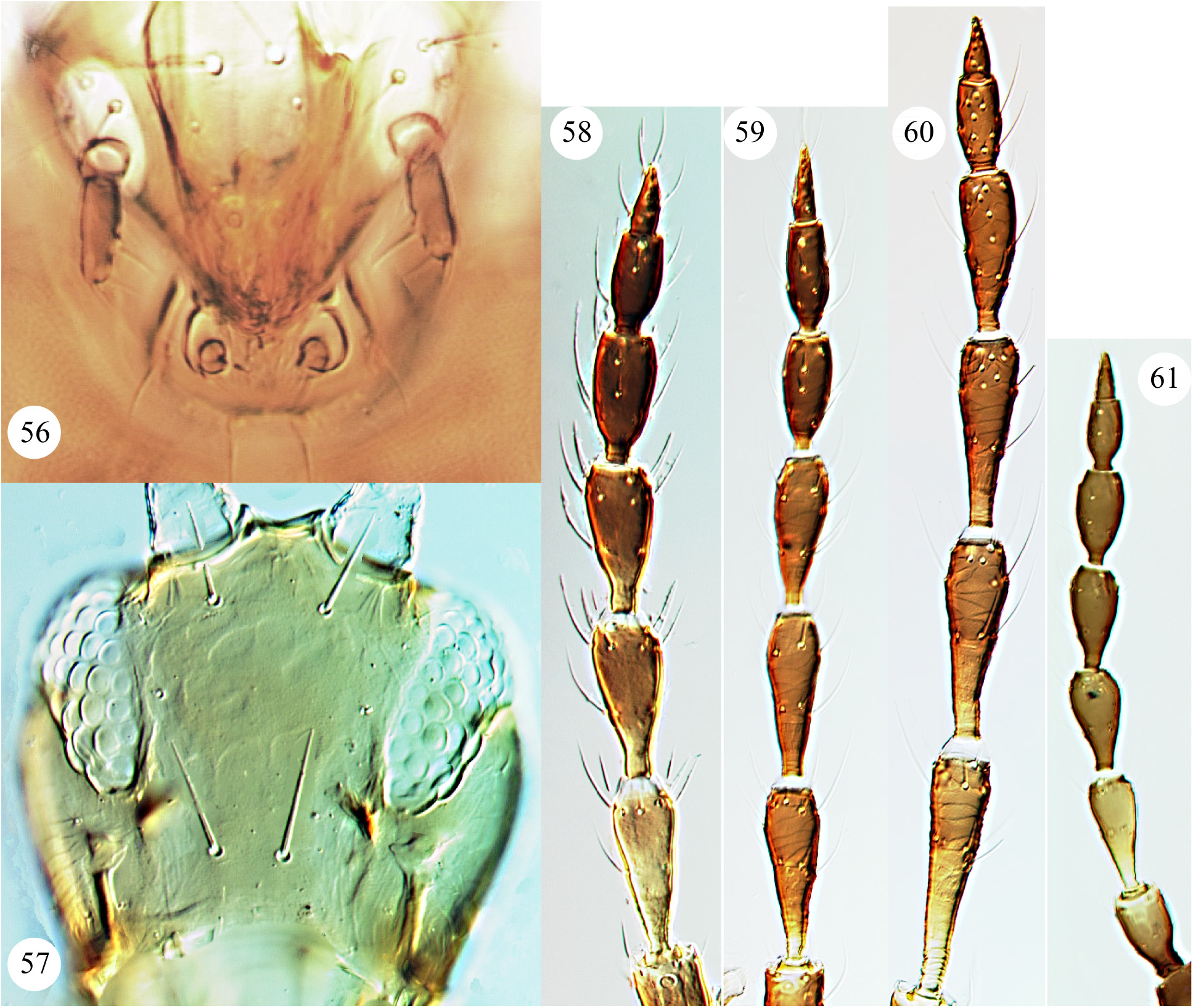
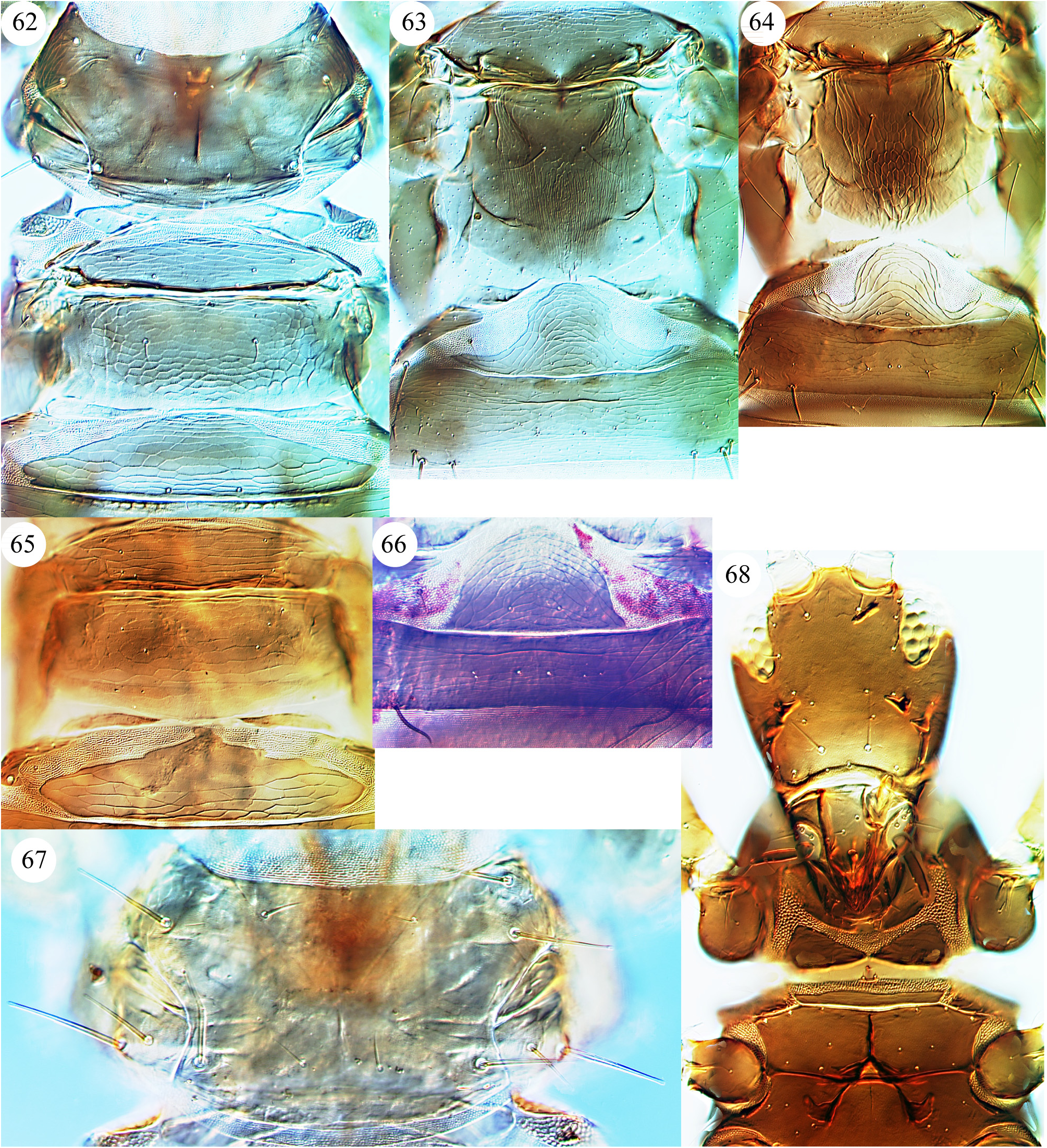
As indicated above in the Introduction, the maxillary palps of Carientothrips species are unusual amongst Phlaeothripidae , in that the basal segment is relatively long and sometimes longer than the second segment which often bears transverse striae. Intra-specific structural variation is confusing within many species of this genus. Part of this variation is sexual, with males commonly having enlarged fore femora, a large fore tarsal tooth, and often an enlarged prothorax with swollen fore coxae, also differences in the form of some setal apices. In females of this genus, the fore tarsal tooth is usually absent, although it is present in the females of C. calami and C. denticulatus . Some character states that have been used to distinguish species previously require careful assessment and are possibly not reliable, these include relative head length, the form of setal apices whether acute, blunt, or weakly capitate, and extent of yellow colour on parts of the body. One character state has proved particularly interesting—the prolongation of the compound eyes on the ventral surface of the head. Mound (1974 a) considered that this varied within the species mjobergi , but that conclusion is rejected here. The ventral prolongation of the eyes in Carientothrips species involves the displacement posteriorly of one (more rarely two or even three) ommatidia along a special groove ( Figs 7–9 View FIGURES 1–9 ). It is not comparable to the eye prolongation found in some other Idolothripinae genera, including Nesothrips propinquus , in which the entire multifaceted posterior margin of the eyes is prolonged ( Figs 57 View FIGURES 56–61 , 68 View FIGURES 62–68 ). Because of the absence of any individuals with the eyes in any suitable intermediate condition, the difference between eyes ventrally prolonged or not prolonged is here interpreted as indicating a difference between species. As a result, four closely similar species are recognised here: alienatus, flavitibia , mjobergi , and tasmanica .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |