Anocellidus profundus, Quiroga, Bolaños & Litvaitis, 2006
|
publication ID |
https://doi.org/10.11646/zootaxa.1317.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5689592 |
|
persistent identifier |
https://treatment.plazi.org/id/03FB3A42-B400-FFBB-FECA-FC8E2494FED8 |
|
treatment provided by |
Plazi |
|
scientific name |
Anocellidus profundus |
| status |
sp. nov. |
Species: profundus View in CoL n. sp. ( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Type material and locality:
a) Holotype, whole mount, one mature specimen ( 10 mm X 10 mm), FMNH 12555 ; collected 11 July, 2003 from 2660 m depth at ODP 1026B ( 47º45.765'N 127º45.439’W) GoogleMaps .
b) Paratype, one mature specimen as serial sagittal sections ( 13 mm X 12 mm), FMNH 12556 ; collected with holotype on 11 July, 2003 from 2660 m depth at ODP 1026B ( 47º45.765'N 127º45.439’W) GoogleMaps .
Other material examined:
c) Whole mount, one mature specimens ( 11 mm X 10 mm), FMNH 12557; collected with holotype on 11 July, 2003 from 2660 m depth at ODP 1026B ( 47º45.765'N 127º45.439’W).
d) One mature specimen, as serial sagittal sections ( 11 X 10 mm), FMNH 12558; collected with holotype on 11 July, 2003 from 2660 m depth at ODP 1026B ( 47º45.765'N 127º45.439’W).
e) One mature specimen, as serial sagittal sections ( 11 mm X 10 mm), FMNH 12559; collected 3 July, 2003 from 2642 m depth at Baby Bare Seamount ( 47° 42.637’N 127° 47.292’W).
f) Ethanolpreserved specimen, not sectioned or mounted, FMNH 11736; collected with holotype
g) Ethanolpreserved specimen, not sectioned or mounted, FMNH 11778; from Baby Bare Seamount.
h) Ethanol preserved specimen, not sectioned or mounted, FMNH 12463; collected on 30 Aug, 2004 from Escanaba Trough, 20 m N of Marker 6X on Central Hill, from 3232 m depth ( 41° 00.272’N 127° 29.679’W).
Etymology: name from an = without, ocell = little eye, for the absence of eyes, and profundus = deep, a reference to the depth at which the specimens were collected.
Distribution: To date, known from the type locality ODP 1026B ( 47º45.765'N 127º45.439’W), from Baby Bare Seamount ( 47° 42.637’N 127° 47.292’W), and from Escanaba Trough ( 41° 00.272’N 127° 29.679’W) at depths from 2642 to 3232 m.
Diagnosis: Male copulatory apparatus posterior to the male pore and directed anteriorly, with a long and pointed stylet directed posterior; prostatic vesicle and prostaticlike glands absent. Welldeveloped, nuchal tentacles, eyes completely lacking. Ventral sensory disk present anterior to the cerebral ganglion.
Description
External features:
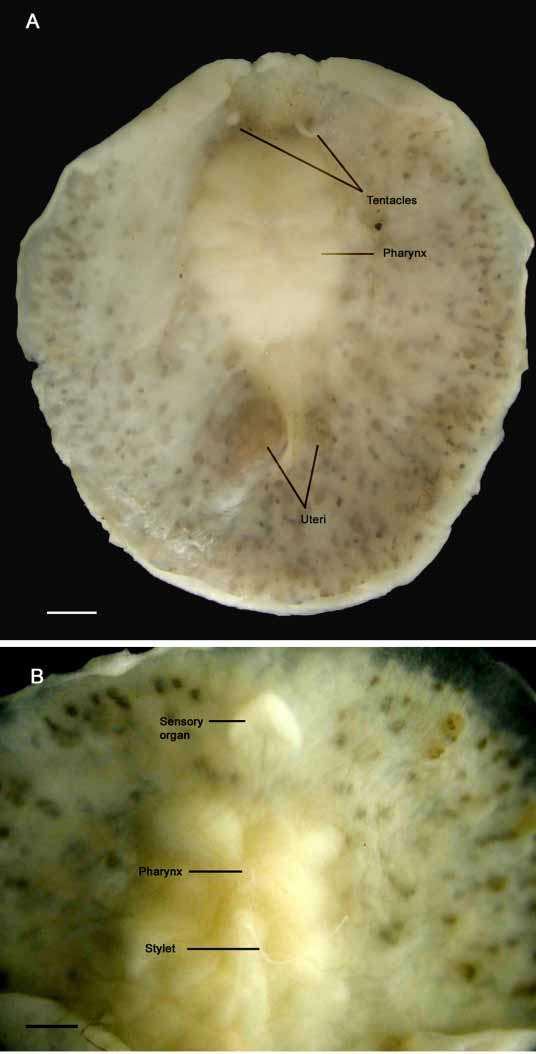
Color — Live and preserved specimens are whitish but testes and ovaries appear as numerous brown dots visible through the epidermis ( Fig. 1 View FIGURE 1 ). In cleared specimens, testes and ovaries arrayed radially about the pharynx; testes appear smaller and dark yellow.
Form — Preserved specimens range from 10 mm x 10 mm to 13 mm x 12 mm and have a fleshy, rounded body ( Fig. 1 View FIGURE 1 A). The body margin is smooth without evident folds. Ventrally, an arrowheadshaped organ of putative sensory function lies just anterior to the cerebral ganglion ( Figs. 1 View FIGURE 1 B, 2, and 3).
Tentacles — long, pointed, nonretractile nuchal tentacles are present ( Fig.1 View FIGURE 1 A).
Eyes — absent.
Digestive system — a ruffled and very muscular pharynx with 45 deep folds on both sides is medial in the anterior third of the body ( Figs. 1 View FIGURE 1 B, 2 and 4). A small mouth is present in the anterior part of the pharynx. In histological sections, a medial intestinal branch extends anteriorly dorsal to the brain and posteriorly dorsal to Lang’s vesicle ( Fig 3 View FIGURE 3 B). Three to four radial intestinal branches are very conspicuous on either side of the pharynx.
Reproductive anatomy:
Gonopores — male gonopore located just posterior to the mouth and ventral to the pharynx; female gonopore positioned medially, immediately posterior to the male spermiducal bulbs.
Male copulatory apparatus — located posterior to the male pore and directed anteriorly; the stylet, however, is recurved ( Fig. 5 View FIGURE 5 ). The prostatic vesicle is lacking and there is no evidence of a prostatoid organ or prostaticlike glands around the ejaculatory duct or of any glandular epithelium in the male tract. A seminal vesicle and vasa deferentia are absent but two highly muscularized spermiducal bulbs are present lateral and slightly posterior to the pharynx. The spermiducal bulbs appear as a Wshape in whole mounts ( Fig. 2 View FIGURE 2 ) and they fuse to form a single ejaculatory duct. The sinuous ejaculatory duct continues into a very long, thin and pointed stylet ( Figs. 1 View FIGURE 1 B, 4, and 5). A very thin, deep stylet pocket parallels the ventral surface of the worm just ventral to the ejaculatory duct ( Fig. 5 View FIGURE 5 ). The male atrium is shallow and the stylet was found extruded in most specimens ( Figs. 1 View FIGURE 1 B and 5).
Female apparatus — shallow female atrium. The vagina is spacious and curves posterior, terminating in a large, elongated Lang’s vesicle ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 B, 4 and 5). The uteri lie on either side of Lang’s vesicle and the oviducts form a loop before joining with the vagina immediately proximal to Lang’s vesicle ( Fig. 4 View FIGURE 4 ).
Reference measurements from the largest ( 13 mm x 12 mm) available specimen: brain, 375 µm in diameter; Lang’s vesicle, 1.75 mm long; pharynx, 3mm long; stylet, 1037 µm X 75 µm; spermiducal bulbs, 300 µm maximum diameter; tentacles, 625 µm long X 250 µm maximum diameter at their base.
Taxonomic remarks
Although the classification system of Prudhoe (1985) is more conservative because it recognizes fewer taxa, the use of Faubel (1983, 1984a) is appropriate because of its importance on characters of the reproductive system. Anocellidus profundus lacks a prostatic vesicle and in the system of Faubel (1983, 1984a), would be placed in the Ilyplanoidea. This superfamily corresponds to the Cestoplanoidea of Prudhoe (1985). Prudhoe (1985) subdivided the Acotylea into three superfamilies depending on the position of eyes, recognizing marginal, tentacular, cerebral, and frontal eyes. Absolute eye positions are often difficult to determine with areas of cerebral, marginal, and frontal eyes overlapping. And although in his lower taxonomic units, Faubel (1983, 1984a) does place importance on what we consider minor or developmentally plastic details (e. g., type of lining of the prostatic vesicle), the presence or absence of an entire structure such as the prostatic vesicle, does appear to be a much more reliable character than the position of eyes.
Even though the reproductive anatomy of Anocellidus profundus is somewhat similar to that of species in the euplanid genus Aprostatum , it clearly does not pertain to that genus ( Table 2 View TABLE 2 ). The presence of nuchal tentacles, the total absence of eyes, and the presence of a unique ventral sensory organ also separate A. profundus from all other members of the Euplanidae ( Table 1 View TABLE 1 ). In fact, current classification interprets the defining character of “orientation of male reproductive complex” in A. profundus as meriting familial status.
Here we note the presence of tentacles in Anocellidus profundus , although the character’s systematic value is uncertain. For example, Faubel (1983) defines the genus Armatoplana as lacking tentacles, however, Armatoplana divae has tentacles ( Faubel 1983), as does A. colombiana ( Bolaños et al., 2006) . Also the absence of eyes in A. profundus may relate more to its environment than to its phylogenetic history.
However, we recognize the ventrally located putative sensory organ anterior to the cerebral ganglion as an unusual character. A cursory examination of this disk may result in its being confused with a cotylean sucker and the mistaken placement of the specimens in the Cotylea. The presence or absence of a true sucker posterior to the female gonopore has defined the suborders Cotylea (with sucker) and Acotylea (without sucker) ( Lang, 1884). However, exceptions exist; species of acotyleans with a sucker include Leptoplana tremellaris (O.F. Müller 1774) and Itannia ornata Marcus 1947 , although these suckers are never positioned behind the female gonopore (the typical sucker position in cotyleans). In L. tremellaris , the sucker is a depression between the male and female gonopore along the midline of the body ( Faubel 1983). In I. ornata , two adhesive organs are posterior to the gonopore on either side of the midline ( Marcus 1952). Despite the presence of such suckers, other characteristics, such as position of the tentacles, structure of the copulatory complex, and arrangement of eyes determine the placement of I. ornata into the Acotylea ( Bock 1913, Hyman 1951, Faubel 1983, 1984, Prudhoe 1985). Similarly, the presence of a Lang’s vesicle and nuchal tentacles in Anocellidus profundus clearly places this species in the Acotylea.
Three noteworthy characteristics of this putative sensory organ of Anocellidus profundus show that it is not homologous with the cotylean sucker. First, lateral nerve cords appear to innervate the organ ( Fig. 3 View FIGURE 3 A), secondly, rhabdites are completely lacking from the organ’s epithelium (a distinct characteristic of cotylean suckers), and finally, the organ is located just subterminal to the anterior margin. From these observations, it appears then that this organ may serve a sensory rather than an adhesive function. However, additional studies are certainly needed to confirm this.
TABLE 2. Comparison of morphological features of species in the genus Aprostatum * (i. e., lacking a prostatic vesicle and / or prostatoid organs) and the new species Anocellidus profundus.
| Species | Pharynx | Eyes | Tentacles |
|---|---|---|---|
| Anocellidus profundus | Anterior with 45 folds | Absent | Long, pointed nuchal tentacles |
| Aprostatum stiliferum * | Numerous folds | Marginal eyes in irregular rows Absent surrounding entire body; numerous small eyes in fanshape over cephalic region | |
| Aprostatum clippertoni | Small, with small lateral folds | As in A. stiliferum | Absent |
| Aprostatum longipenis | Morphologically uniform Present | Absent | |
| FMNH |
Field Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |