Lasiodora C. L. Koch, 1850
|
publication ID |
https://doi.org/10.11646/zootaxa.5390.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:7C213C54-9AED-45E6-AC69-9E9DF97019A3 |
|
DOI |
https://doi.org/10.5281/zenodo.10470852 |
|
persistent identifier |
https://treatment.plazi.org/id/03FB87A1-FF88-FFA2-99CA-FAEEFA4BFBA6 |
|
treatment provided by |
Plazi |
|
scientific name |
Lasiodora C. L. Koch, 1850 |
| status |
|
Lasiodora C. L. Koch, 1850 View in CoL View at ENA
( Figs 1 View FIGURES 1–2. 1 ─284)
Mygale : C. L. Koch 1841: 25, f. 708 (in part: M. klugii ).
Lasiodora C. L. Koch 1850: 72 View in CoL ; Simon 1864: 66 (subgenus of Eurypelma ); 1892a: 156, 157, 160; 1903: 917, 926, 937 ─ 939; Ausserer 1871: 128, 208 (subgenus of Eurypelma ); Pocock 1901: 543, 544; Petrunkevitch 1911: 76; 1928: 81; Mello-Leitão 1921: 337; 1923: 220, 242, 271, 386; 1943: 257; Roewer 1942: 250; Bonnet 1957: 2354; Schiapelli & Gerschman de Pikelin 1967: 481, f. 9─15 [misidentification of Theraphosa apophysis (Tinter) View in CoL ]; 1979: 290─291, 294, f. 1─7; Brignoli 1983: 138; Raven 1985: 119; Pérez-Miles et al. 1996: 36 ─37, 40, 42─43, 52, f. 27; Bertani 2001: 283, f. 27, 58─61, 164─165, 167, 174; World Spider Catalog 2023.
Acanthoscurria View in CoL : Mello-Leitão 1923: 297 (in part: A. cristata ).
Type species: Mygale klugi C. L. Koch, 1841 , by subsequent designation ( Simon 1892a: 160).
Species included: Lasiodora klugi (C. L. Koch, 1841) View in CoL , L. benedeni Bertkau, 1880 View in CoL , L. parahybana Mello-Leitão, 1917 View in CoL , L. subcanens Mello-Leitão, 1921 View in CoL , L. camurujipe n. sp., L. sertaneja n. sp., L. franciscana n. sp.
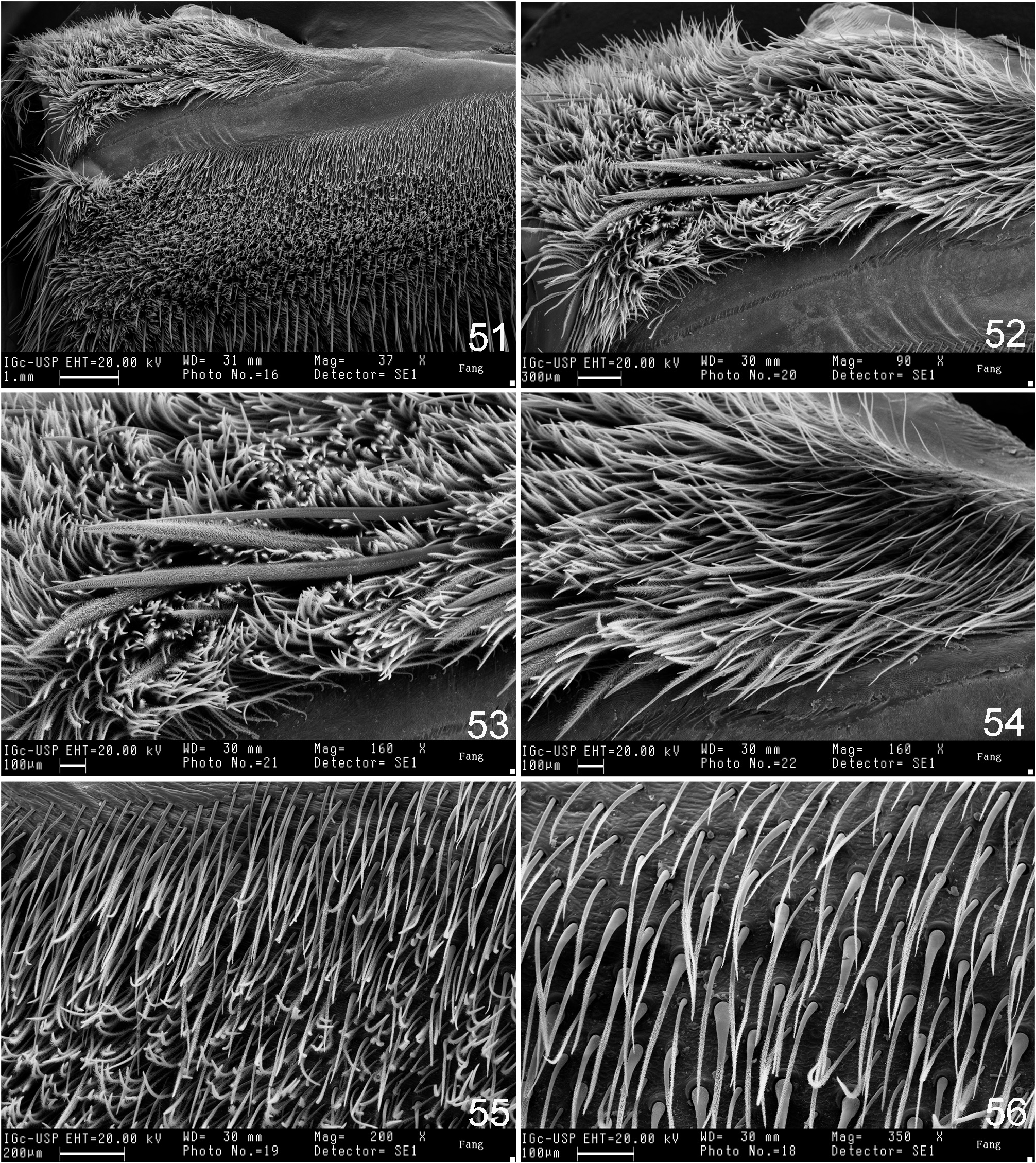
Diagnosis: Male resemble those of species of Vitalius , Nhandu , Crypsidromus , Tekoapora , Pterinopelma , Lasiocyano and Eupalaestrus anomalus by having the embolus with a triangular shaped SA keel ( Figs 3 View FIGURES 3–7 ─4). Female resemble those of Vitalius , Nhandu , Tekoapora , Pterinopelma , and Lasiocyano by having type I urticating setae on the abdomen dorsum in conjunction with spermathecae fused on a short area on their base (Fig. 32). Both males and females can be distinguished from those of the species above by having stridulatory apparatus formed by plumose setae on the distal median area of prolateral coxae I─IV, more developed on coxae I─II ( Figs 51 View FIGURES 51 ─53).
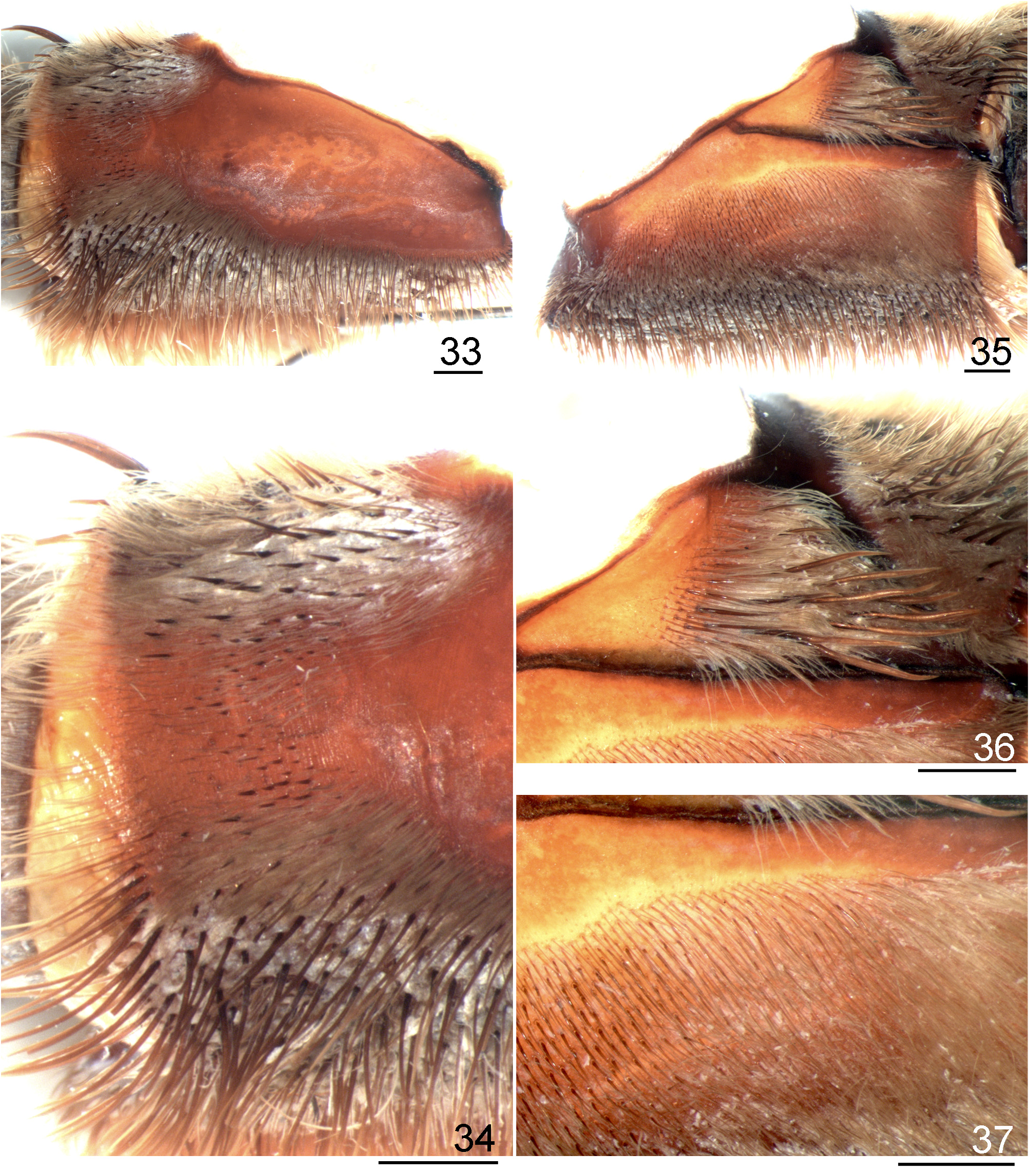
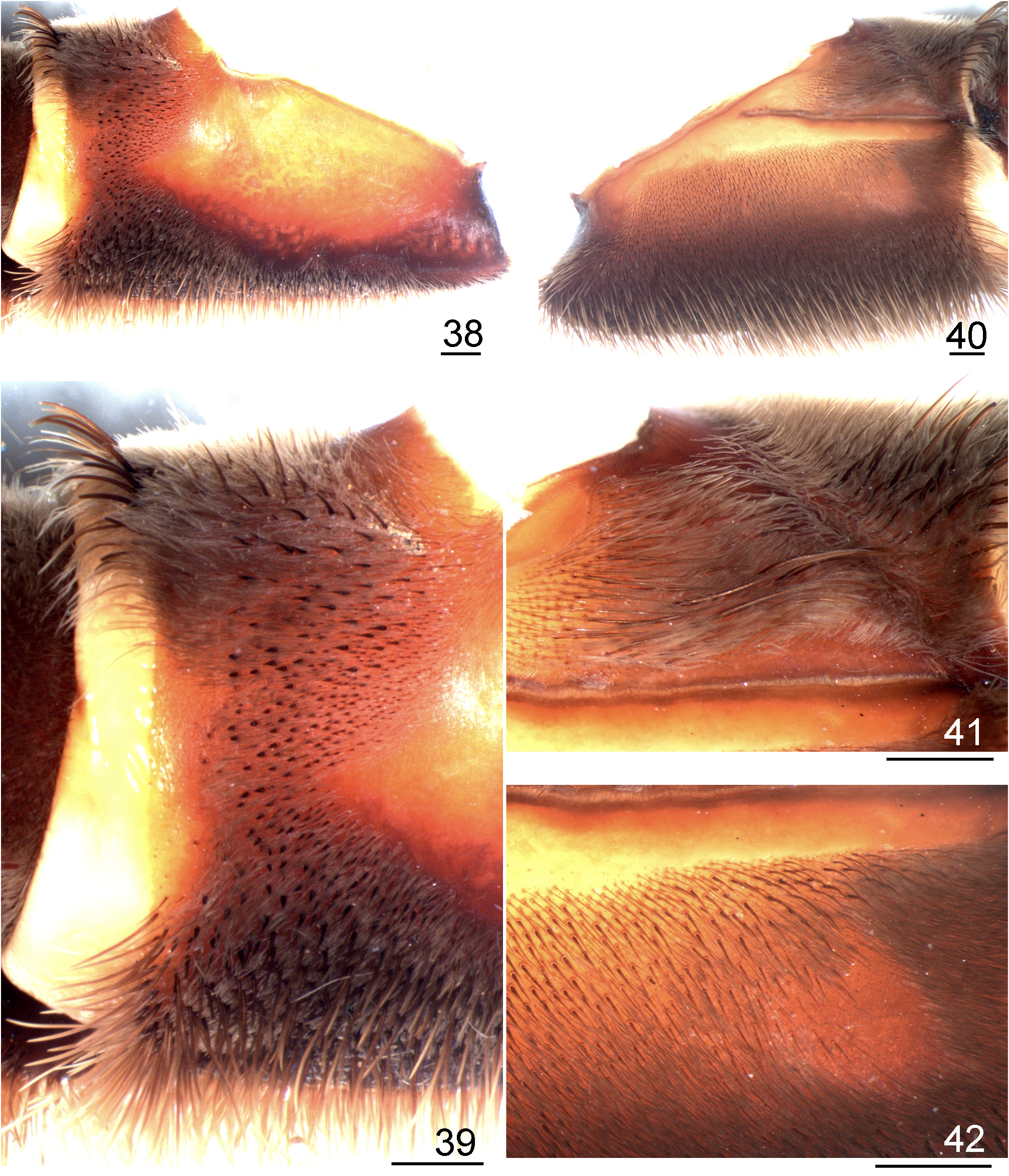
Redescription: Carapace slightly longer than wide, cephalic area moderately (males) to noticeably (females) raised. Cephalic and thoracic striae conspicuous. Fovea deep, straight. Carapace covered with short, slender, dense setae, bordered with long setae pointing out. Chelicerae lacking rastellum, basal segments with 9–15 teeth and denticles on basal area. Eye tubercle distinct, wider than long. Clypeus short. Anterior eye row procurved, posterior recurved or slightly recurved. AME rounded, about same size as ALE and PLE, which are oval in shape. PME small, oval. Labium subquadrate, slightly wider than long, with numerous (103─254) cuspules on its anterior half. Maxilla subrectangular, anterior lobe distinctly produced into a conical process, inner angle bearing numerous cuspules (231─403). Lyra absent. Sternum longer than wide with two large sigilla or a single merged sigillum on the sternum/labium edge; posterior angle rounded, not separating coxae IV. Sigilla: first pair rounded; second and third ovals; all one diameter from margin. PMS one-segmented, short; PLS three-segmented, apical segment digitiform. Leg tarsi lacking spines, claw tufts present. STC with a median row of few small teeth. ITC absent from all legs. Clavate trichobothria on distal 2/3 of tarsi I–IV. Tarsi I–IV and metatarsus I fully scopulated; metatarsus II 3/4 to fully scopulated; metatarsus III scopulated along half its length; metatarsus IV 1/6 to 1/3 scopulated. Retrolateral femur IV with plumose setae. Stridulatory apparatus formed by spiniform setae on retrolateral maxilla ( Figs. 131 View FIGURES 131 ─132) and sometimes on retrolateral coxae I─III and plumose setae on prolateral coxae I–IV, more developed on coxae I─II (Figs. 133─134). Retrolateral maxillae with small to large spiniform setae distributed only on the distal upper region ( L. parahybana , L. benedeni , L. franciscana n. sp.) ( Figs 131 View FIGURES 131 ─132), on the full retrolateral area ( L. subcanens , L. camurujipe n. sp.) ( Figs 176 View FIGURES 176 ─177) or having intraspecific variation ( L. klugi , L. sertaneja n. sp.) ( Figs 38 View FIGURES 38 ─39, 43, 45, 47, 49). Prolateral coxae with acicular ( L. franciscana n. sp., L. sertaneja n. sp.) or spatulate plumose setae ( L. klugi , L. benedeni , L. parahybana , L. subcanens , L. camurujipe n. sp.) ( Figs 8 View FIGURES 8–15 ─15). Male tibial spur with two non-converging processes originating from common base. retrolateral longest and with a curvature at its distal portion (Figs 30─31). Metatarsus I curved on its basal third; when folded, it touches the apex of the retrolateral tibial process. Male palpal bulb pyriform, embolus slightly flattened distally. Embolus: PS and PI keels present, the PS forming the embolus edge distally. R keel sharp. A keel short. SA keel well developed ( Figs 3 View FIGURES 3–7 ─4). Spermathecae short, separated by a heavily sclerotized short area (Fig. 32). Spermatheca stalk narrower than spermatheca bulb. Type I and III urticating setae present in male and female. Type I normally on MA, LA, LM, LP areas and type III on MM and MP areas (as in Bertani & Guadanucci 2013, fig. 7).
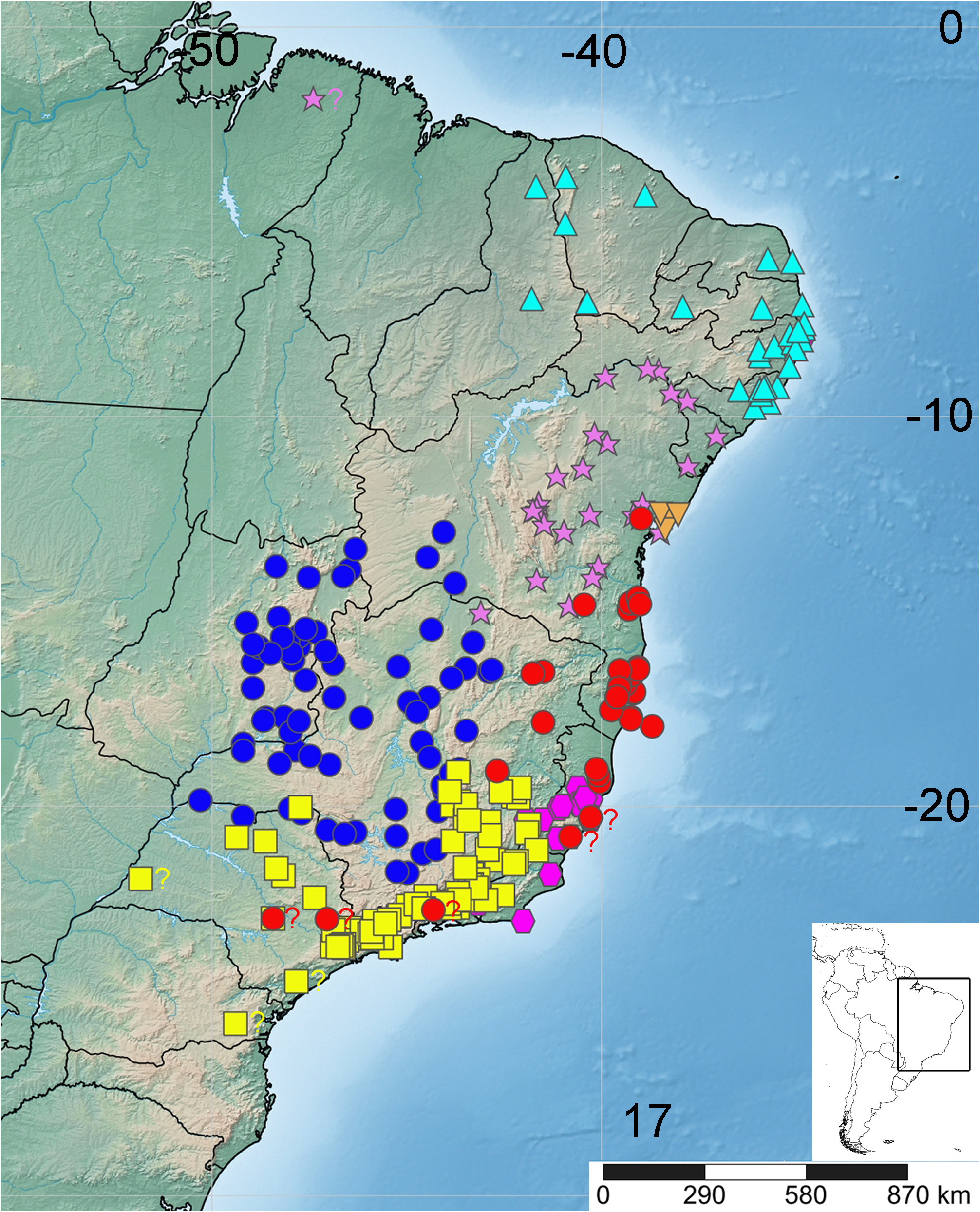
Distribution and notes on natural history. The genus Lasiodora is endemic to Brazil, occurring from states of Piaui and Ceará in the north, to the state of São Paulo, in the south; state of Goias in the west; and the coast, from states of Piaui and Ceará to state of Rio de Janeiro in the east ( Figs 17 View FIGURE 17 ─18). Specimens of Lasiodora are sometimes found in shipments of wood or other goods, traveling to areas where they do not naturally occur (pers. obs.). Thus, some records from other areas distinct from the above are questionable.
Lasiodora species are distributed mainly on areas of Brazilian Atlantic Forest on the coast, but also in the interior of Brazil, in the biomes of Cerrado and Caatinga. In these two savannah-like (Cerrado) or semiarid (Caatinga) biomes, Lasiodora seems to occur in small patches of forested areas, such as riparian or hillside forests. For example, Lasiodora parahybana specimens are common on the rainforest of the Northeast coast, but there are records for the species in the Caatinga biome. Some areas in the Caatinga, known locally as “brejos de altitude”, are relicts of an ancient connection, in more humid periods, between the Amazon Forest on the north and the Brazilian Atlantic Forest on the east coast ( Ab’Saber 1977; Carnaval & Moritz 2008). After a drier period, that connection was disrupted and most areas are now covered with caatinga vegetation. On more elevated regions, however, patches of Atlantic Forest remained, surrounded by semiarid areas, isolating populations of Lasiodora parahybana .
The dependency to humid environments, perhaps, explain why it is common to find Lasiodora specimens inside caves ( Trajano 2000). Caves are normally colder and wetter than the external environment, mainly those in the Cerrado and Caatinga, and can be used as retreats by the spiders. Vitalius is another genus having species living in similar habitat as those of Lasiodora , that is, the Brazilian Atlantic Forest. However, they are common in Southeastern Brazil, where semiarid regions as those of Caatinga are lacking and there are only small patches of Cerrado. In this more humid region, Vitalius species, as other theraphosids, were never recorded inside caves, even though the fauna of several caves of that region are well explored and studied ( Trajano 2000). One reason could be the contrasting external environments of the two cave regions.
Key to Lasiodora species
1 Distal retrolateral maxilla with spiniform setae on upper, median, and sometimes, lower areas ( Figs 38 View FIGURES 38 ─39)............. 2
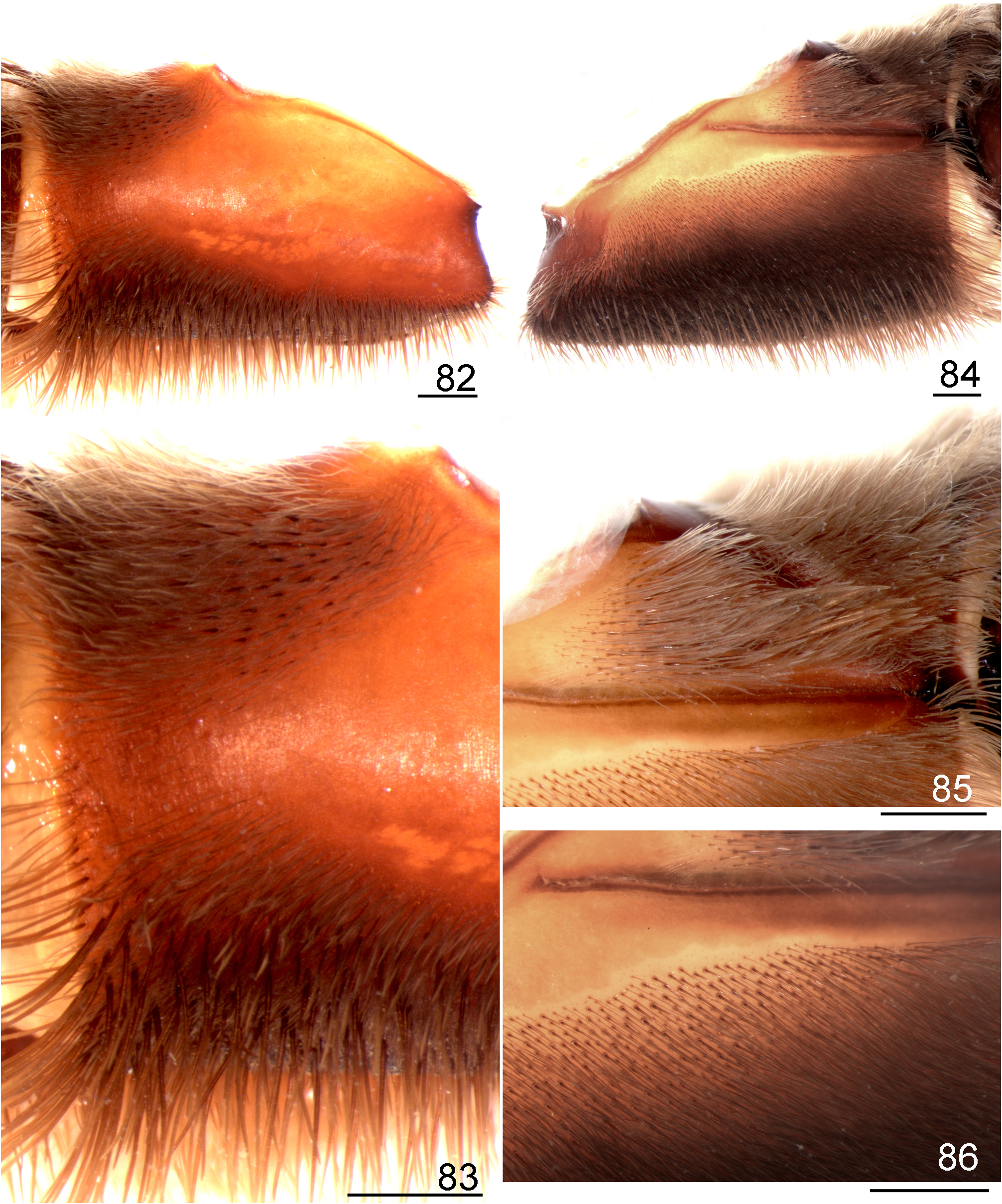
- Distal retrolateral maxilla with spiniform setae only on upper area ( Figs 82 View FIGURES 82 ─83)................................... 5
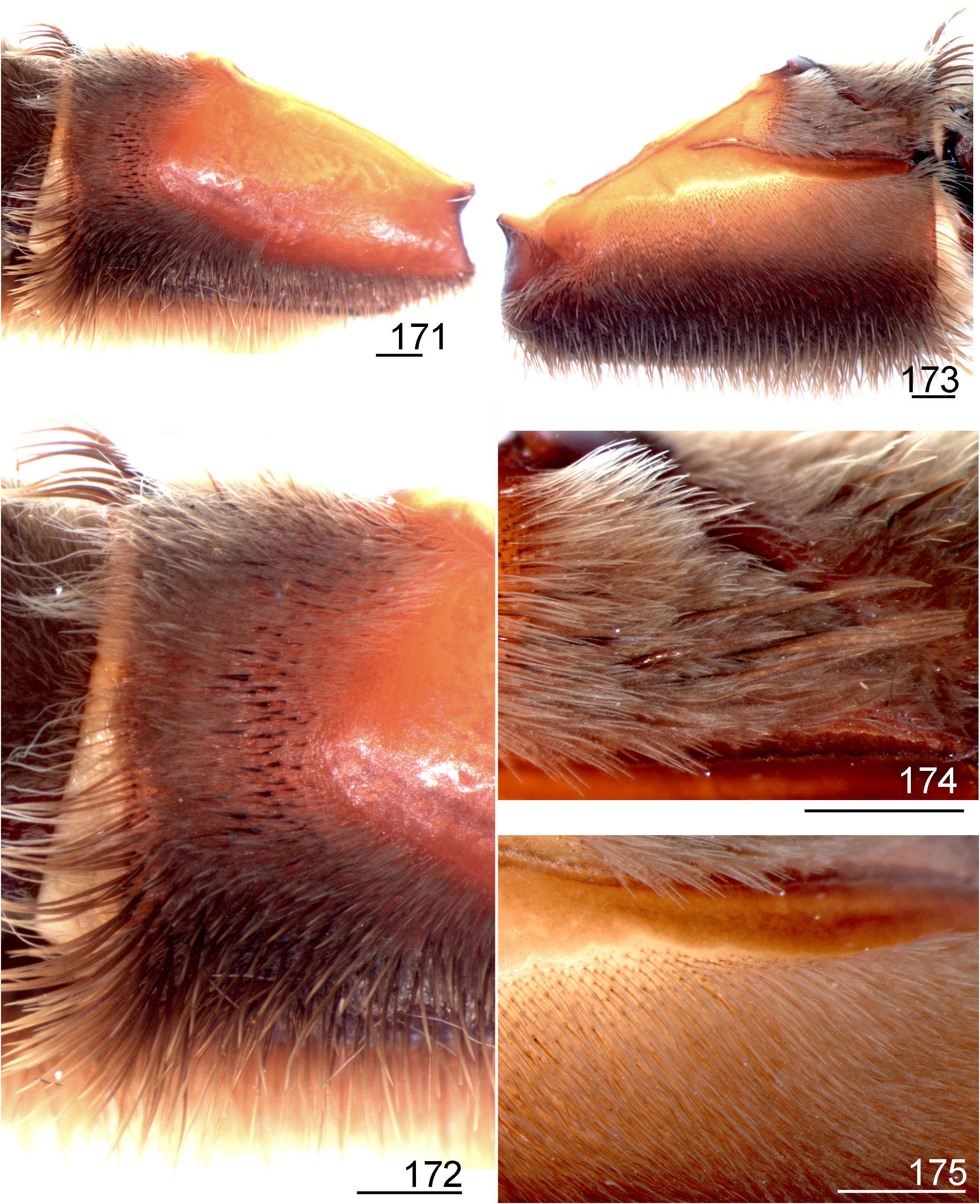
2 Retrolateral distal maxilla always covered with several spiniform setae on most of its upper, median and lower areas ( Figs 171 View FIGURES 171 ─172); stridulatory setae on coxa I short, normally spatulate or almost so (Figs 173─174)......................... 3
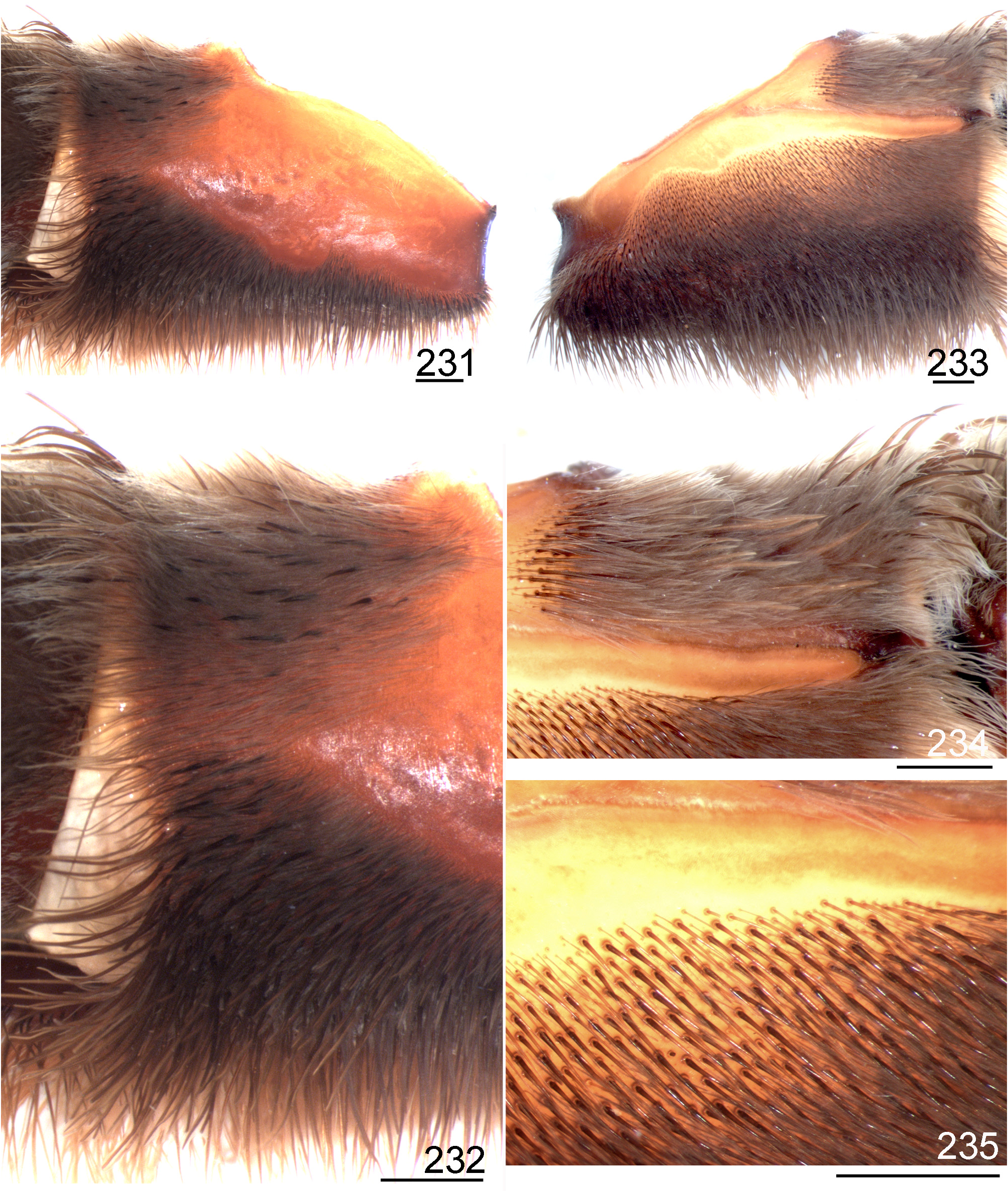
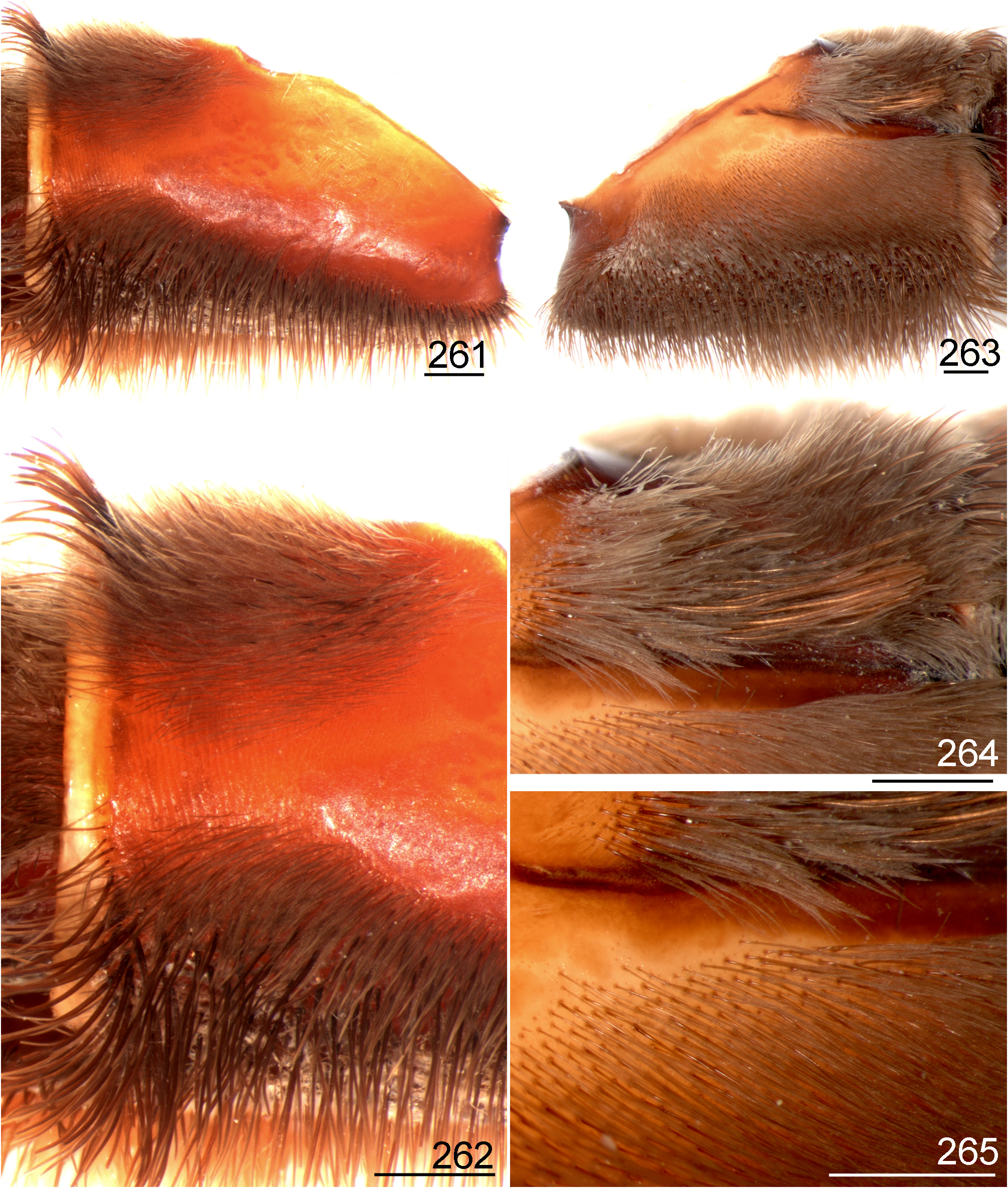
- Retrolateral distal maxilla with spiniform setae concentrate on its upper area, median and lower areas normally with few spiniform setae irregularly distributed ( Figs 231 View FIGURES 231 ─232, 241, 243); stridulatory setae on coxa I long, slender, acicular or slightly spatulate (Figs 263─264)............................................................................... 4
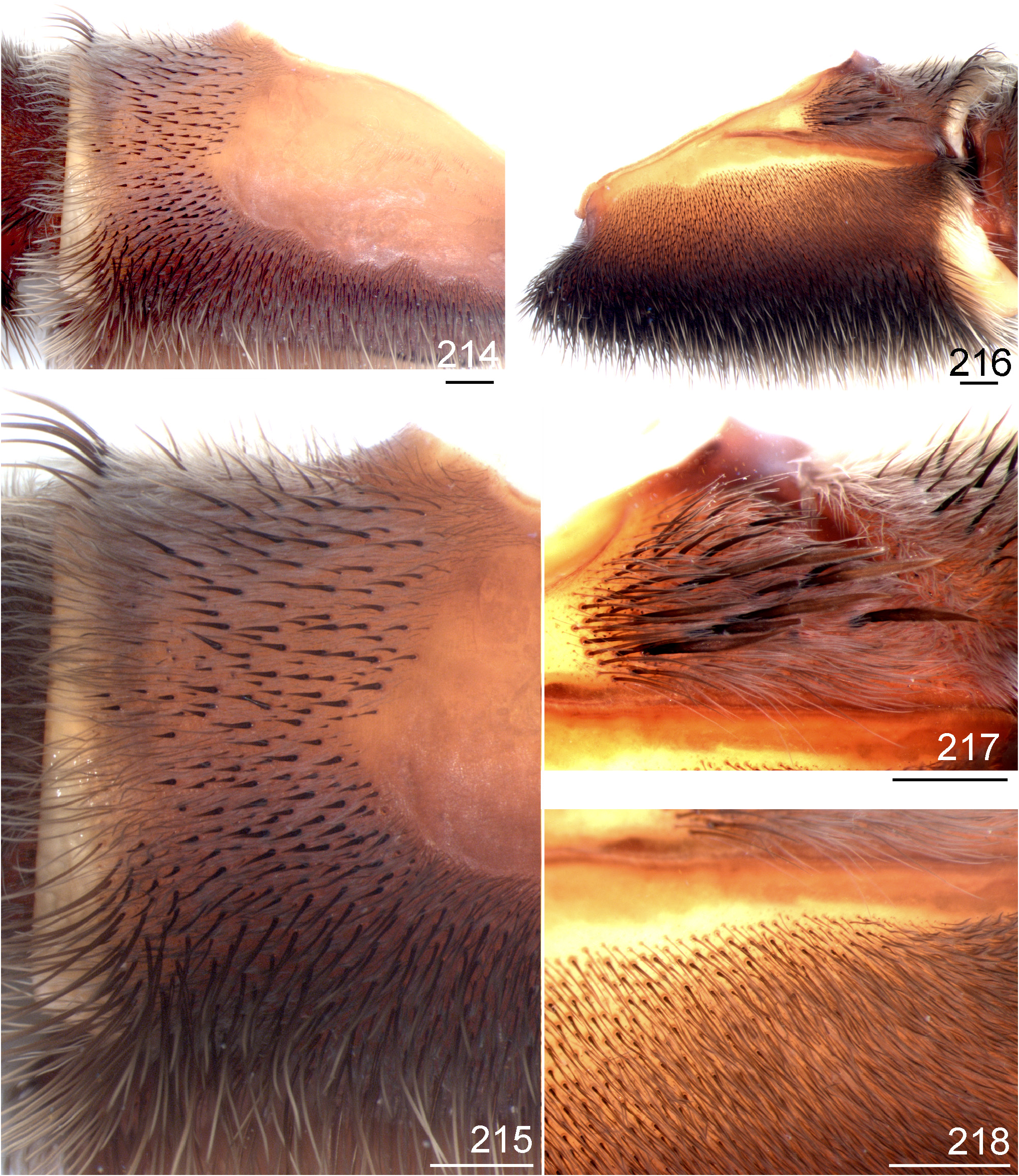
3 Retrolateral distal maxilla covered with several well developed spiniform setae ( Figs 214 View FIGURES 214 ─215); carapace and dorsal chelicerae and legs with few whitish long setae ( Figs 219 View FIGURES 219 ─222).......................................... L. camurujipe n. sp.
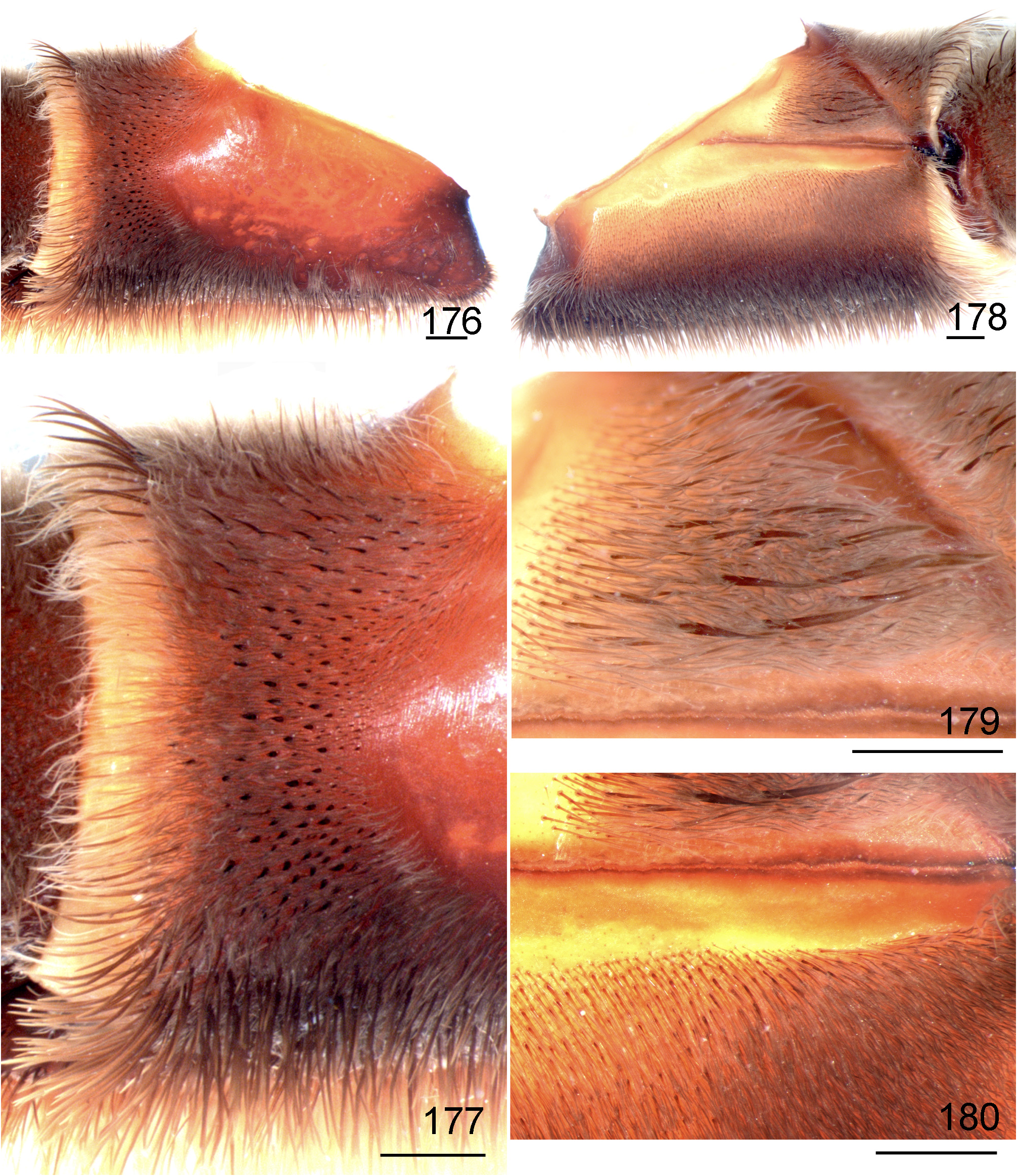
- Retrolateral distal maxilla covered with several short spiniform setae ( Figs 176 View FIGURES 176 ─177); abundant whitish setae covering most carapace, dorsal chelicerae and legs ( Figs 199 View FIGURES 199 ─200)................................................. L. subcanens View in CoL
4 Retrolateral distal maxilla covered with long spiniform setae on its upper area, lower and median areas with some scattered long spiniform setae (Figs 237, 241, 243); stridulatory setae on prolateral coxae very slender, acicular, slightly plumose ( Figs 9 View FIGURES 8–15 , 239); lower areas of prolateral coxa I sometimes with setae having very incrassate base (Fig. 240); male bulb apex slightly incrassate ( Figs 225 View FIGURES 225 ─227).......................................................... .. L. sertaneja n. sp. (part).
- Retrolateral distal maxilla covered with short spiniform setae on its upper area, lower and median areas with some scattered spiniform setae ( Figs 33 View FIGURES 33 ─34); stridulatory setae on prolateral coxae slightly spatulate, plumose, usually long ( Figs 13 View FIGURES 8–15 , 35─36); lower areas of prolateral coxa I with setae having non incrassate or slightly incrassate bases (Fig. 37); male bulb apex non incrassate ( Figs 27 View FIGURES 27 ─29)....................................................................... L. klugi View in CoL (part).
5 Stridulatory setae on prolateral coxae short, spatulate ( Figs 8 View FIGURES 8–15 , 133─134)................................ L. parahybana View in CoL
- Stridulatory setae on prolateral coxae slender, acicular or slightly spatulate (Figs 263─264)...........................6
6 Retrolateral distal maxilla covered with weakly developed spiniform setae on upper area ( Figs 261 View FIGURES 261 ─262)............... 7
- Retrolateral distal area covered with developed spiniform setae on upper area (Figs 232, 241, 243)..................... 8
7. Male palp bulb: embolus thickened, distal portion thick ( Figs 255 View FIGURES 255 ─257); spermatheca bulb enlarged (Fig. 260)................................................................................................ L. franciscana n. sp.
- Male palp bulb: embolus slender, distal portion slender ( Figs 76 View FIGURES 76 ─78); spermatheca bulb slender (Figs 81, 120)... L. benedeni View in CoL
8. Stridulatory setae on prolateral coxae acicular ( Fig. 9 View FIGURES 8–15 , 239); lower areas of prolateral coxa I sometimes with setae having very incrassate base (Fig. 240); male bulb embolus apex incrassate ( Figs 225 View FIGURES 225 ─227); spermatheca bulb incrassate (Fig. 230)..................................................................................... .. L. sertaneja n. sp. (part).
- Stridulatory setae on prolateral coxae slightly spatulate, long ( Fig. 13, 15 View FIGURES 8–15 , 36); male palpal bulb embolus distal portion not incrassate ( Figs 27 View FIGURES 27 ─29); spermatheca bulb not incrassate (Fig. 32)..................................... L. klugi View in CoL (part).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Lasiodora C. L. Koch, 1850
| Bertani, Rogério 2023 |
Acanthoscurria
| Mello-Leitao, C. F. 1923: 297 |
Lasiodora C. L. Koch 1850: 72
| Bertani, R. 2001: 283 |
| Perez-Miles, F. & Lucas, S. M. & Silva Jr., P. I. & Bertani, R. 1996: 36 |
| Raven, R. J. 1985: 119 |
| Brignoli, P. M. 1983: 138 |
| Schiapelli, R. D. & Gerschman de Pikelin, B. S. 1967: 481 |
| Bonnet, P. 1957: 2354 |
| Roewer, C. F. 1942: 250 |
| Petrunkevitch, A. 1928: 81 |
| Mello-Leitao, C. F. 1923: 220 |
| Mello-Leitao, C. F. 1921: 337 |
| Petrunkevitch, A. 1911: 76 |
| Pocock, R. I. 1901: 543 |
| Ausserer, A. 1871: 128 |
| Simon, E. 1864: 66 |
| Koch, C. L. 1850: 72 |
Mygale
| Koch, C. L. 1841: 25 |