Pavlova Butcher emend. Véron
|
publication ID |
https://doi.org/10.5852/ejt.2023.861.2063 |
|
DOI |
https://doi.org/10.5281/zenodo.7713655 |
|
persistent identifier |
https://treatment.plazi.org/id/03FBF116-8460-FFF6-FDA0-FE7969CDFEE5 |
|
treatment provided by |
Felipe |
|
scientific name |
Pavlova Butcher emend. Véron |
| status |
|
Genus Pavlova Butcher emend. Véron View in CoL View at ENA
Figs 10–13 View Fig View Fig View Fig View Fig
The unidentified strains of Pavlova (AC248 and AC250) were chosen for study because none had ever been examined within the sub-clade 3.2 ( Bendif et al. 2011); both strains were found to have the same cytomorphological characteristics as the genus Pavlova .
Description of strains AC248 and AC250
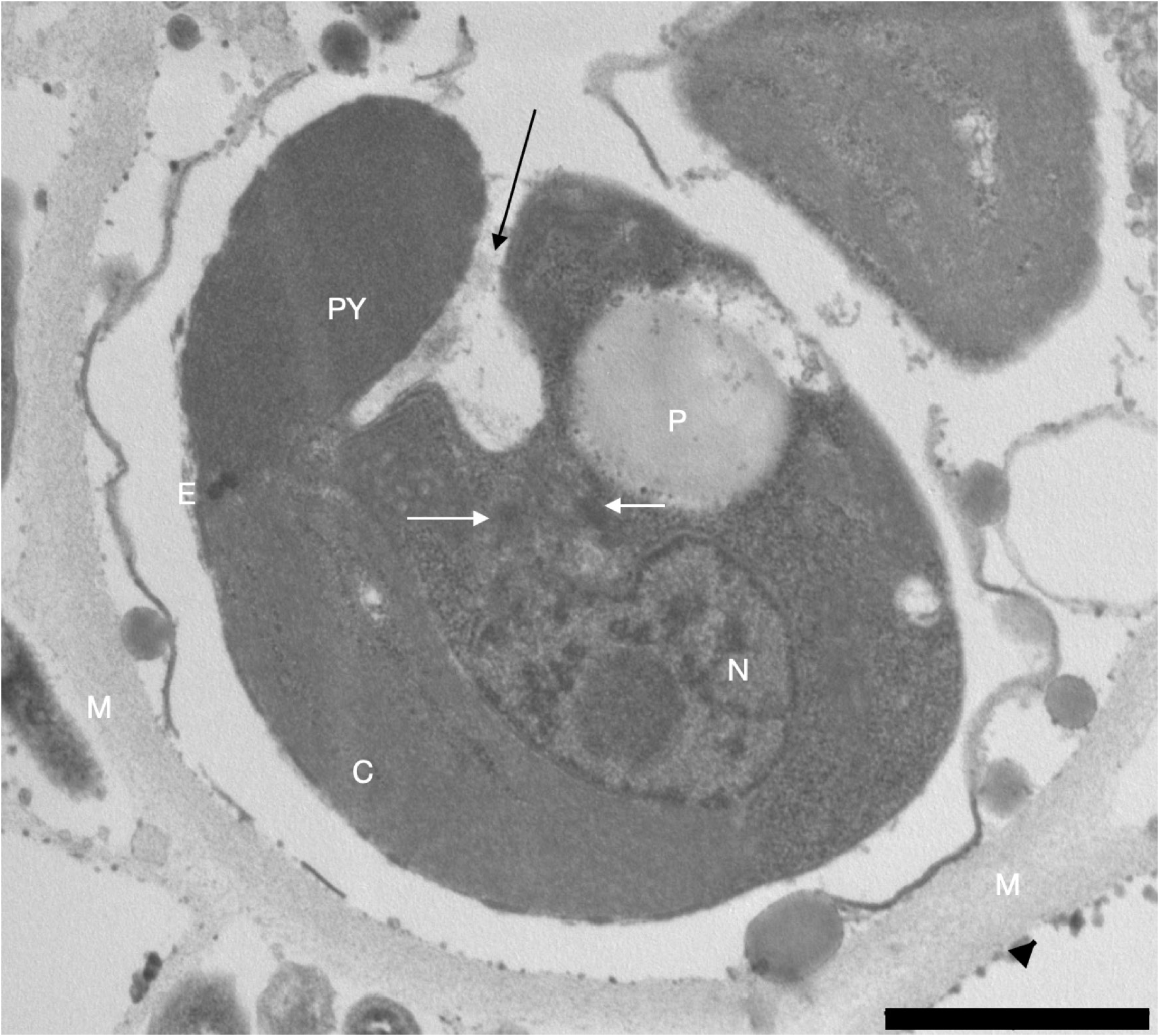
Non-motile cells are occasionally present in cultures, clustered to a few in a loose mucilage ( Fig. 11A View Fig ) and showing a reduced flagellar appendage. Motile cells are slightly ovoid (5.9 µm ± 0.5 × 5.2 µm ± 0.4, n = 49), free swimming and highly metabolic ( Fig. 10 View Fig ). Emergence of the appendages is from a narrow, shallow sub-apical pit ( Fig. 12A View Fig ). Except at its base ( Fig. 12E View Fig ), the AF (9.5 µm ± 3.8, n = 12) is coated with several layers of regularly spaced ( Fig. 12D View Fig ) flat and ovoid KS (≈ 47 × 34 nm, n = 4) with a slight median constriction and with fine non-tubular hairs ( Fig. 12C View Fig ). The smooth and short PF (1.9 µm ± 0.5, n = 6) is tapered distally ( Fig. 12B View Fig ). The bipartite H (1.1 µm ± 0.2, n = 7) consists of a proximal part of constant diameter and a distal part of equal length and smaller diameter. The single cup-shaped parietal C ( Fig. 11B View Fig ) contains bundles of thylakoids grouped in stacks three to five ( Fig. 11C View Fig ). One end of the C, near the pit and F bases, contains a conspicuous orange E ( Fig. 10A–B View Fig ) consisting of a cluster of osmiophilic globules located along its inner surface ( Fig. 11B, D View Fig ). In the centre of the C, opposite the F base, is a PY forming an ovoid bulge at the cell surface ( Figs 10C View Fig , 11B View Fig , 12A View Fig ). In transverse section, this protruding PY has the unusual aspect of a thick, wide utricle ( Fig. 13A–B, D View Fig ) curving in on itself ( Fig. 13B–C View Fig ) and entirely surrounded by the C-membrane bordered by the periplastic ER.
Details of the pyrenoid of Pavlova spp.
This particular form of PY, present in strains AC248, AC250 and also AC33 ( Fig. 13D View Fig ), had previously been observed in various species of Pavlova (i.e., P. pinguis , P. gyrans and P. granifera ) but had not been retained as a marker of the genus. It turns out that with all strains of Pavlova for which we now have sections, this PY is a very distinctive feature of the genus.
Indeed, at the time of the revision of the species P. pinguis, Green (1980) observed this pyrenoid very clearly in posterior position which he described as “large and conspicuous, frequently being pushed into a bulge at the posterior end of the cell.”. He also noted that this PY is “...frequently penetrated by a tubular invagination containing cytoplasmic material...”. In fact, his illustrations (see his figs 8, 44, 45) clearly show the curved shape of this PY in P. pinguis , as does fig. 7F of Bendif et al. (2011). In their revision of P. gyrans, Green & Manton (1970) noted the central position of the PY within the C as well as its prominent bulging shape but did not examine thin-sections in TEM allowing them to see its curved shape. In their revision of the genus Pavlova they retained the fact that the C is bilobed with a prominent PY. Bendif et al. (2011) also showed this recurving PY in P. gyrans (see their fig. 6G) but retained only the bulge it forms on the cell. For P. granifera, Green (1973) showed the same shape and organisation of the PY (see his fig. 4) with an extension this time towards the interior of the cell (see his fig. 35), a situation we also observed only in the case of Pavlova AC 250 ( Fig. 13C View Fig ). Green (1973) did not retain the singular shape of this PY but reports in his revision of the P. granifera , that the PY is “discretely bulging towards the interior of the cell” ( Green 1980). Bendif et al. (2011) also observe this pyrenoid in P. granifera but with less detail.
The singular shape of this PY that we describe as campylotropous is indeed a distinctive feature of the species of the genus Pavlova since in Exanthemachrysis (the other genus of Pavlovophyceae with a bulging pyrenoid) the two species now described do not show such a recurving shape, but a simple sphero-ovoid PY (see for E. gayraliae: Gayral & Fresnel 1979 : figs 11–12, 21–22 and Bendif et al. 2011: fig. 4E; for E. fresneliae sp. nov.: Fig. 3 View Fig ).
When Butcher (1952) erected the genus Pavlova , he noted the presence of “leucosin bodies” in the posterior part of the cells and his drawings (see Butcher 1952: pl. II, figs 35–37) clearly show what is now known to be this type of PY. He did not retain this character as distinctive of the genus, nor did Green (1967), as cited above, when describing P. pinguis and his subsequent revision of the genus Pavlova . Bendif et al. (2011) introduced a more detailed description of the single C in their revised description by stating that it had a “posterior bulging pyrenoid and conspicuous eyespot on the inner surface near the flagellar pit”.
Taxonomic outcome: a revised description of Pavlova
The particular and very characteristic shape of the pyrenoid in all species of Pavlova makes it a very distinctive feature that leads us to a revision of the genus description.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |