Aspidoras albater Nijssen & Isbrucker, 1976
|
publication ID |
https://doi.org/10.1590/S1679-62252011005000045 |
|
persistent identifier |
https://treatment.plazi.org/id/03FCC70B-1D1A-FFC8-FCF6-FF69A22D0398 |
|
treatment provided by |
Carolina |
|
scientific name |
Aspidoras albater Nijssen & Isbrucker, 1976 |
| status |
|
Aspidoras albater Nijssen & Isbrucker, 1976 View in CoL
Aspidoras albater Nijssen & Isbrucker, 1976: 115 View in CoL , fig. 6. ( Type locality: Brazil, Goiás, rio Tocantizinho, near São João da Aliança GoogleMaps , 14°46’S 47°30’W, rio Tocantins system).
Description. Comparative morphometric and meristic data shown in Table 1. General body shape in Figs. 1 - 2 View Fig View Fig . Head robust, with dorsal profile nearly straight from snout tip to eye and slightly convex from that point to supraoccipital tip. Head and snout roughly triangular in dorsal aspect. Dorsal profile of body straight to slightly convex from tip of supraoccipital process to dorsal fin. Profile nearly straight to slightly concave from dorsal-fin spine to adipose fin; slightly concave from that point to caudal-fin base. Ventral profile straight to slightly convex from isthmus to anal-fin origin; slightly concave from that point to caudal-fin base.
Orbit highly variable among specimens, from nearly normal sized to completely obliterated by frontal, sphenotic, and infraorbital bones. Eyeball usually reduced in size, not visible externally, sometimes visible through bones when orbit obliterated. Mouth subterminal, approximately equal to internarial distance. Two pairs of maxillary and one pair of mental barbels.
Nasal, frontal, sphenotic, compound pterotic, and supraoccipital bones visible externally, devoid of odontodes. Fontanel ovoid and small, covered by thin skin and delimited only by frontal, not reaching supraoccipital. Supraoccipital fossa very small and covered by thin skin. Supraoccipital roughly quadrangular, with short posterior process separated from dorsal-fin nuchal plate by three dorsolateral body plates. Two infraorbital bones covered by thin skin and visible externally. Opercle visible externally, without odontodes. Preopercle covered by thin skin and visible externally; interopercle not visible externally, covered by thick skin.
Trunk lateral line reduced to two latero-sensory ossicles. Two series of elongate plates covering entire body. Nuchal plate covered by skin and not visible externally. Three to eight azygous plates anterior to adipose-fin spine. All plates with several minute odontodes restricted to posterior border. Cleithrum and coracoid covered by skin ventrally; cleithrum visible laterally. Dorsolateral body plates 26-27; ventrolateral body plates 23-24. Abdomen entirely covered by scattered odontodes from isthmus to preanal area in adults; odontodes restricted to middle of abdomen in smaller specimens.
Dorsal fin nearly triangular, its origin just posterior to third dorsolateral plate. Ossified portion of dorsal-fin spine shorter than all branched rays; its posterior border smooth. Adipose fin triangular; its origin separated from base of last dorsal-fin ray by 9-10 dorsolateral body plates.Anal fin triangular, origin just posterior to 13 th- 15 th ventrolateral body plate. Pectoral fin ovoid in shape, origin just posterior to gill opening. Ossified portion of pectoral-fin spine shorter than all branched pectoral- fin rays. Inner border of pectoral-fin spine slightly serrated. Pelvic fin ellipsoid, origin just posterior to third ventrolateral body plate, at vertical through base of third or fourth dorsal-fin ray. Caudal fin bilobed, both lobes with approximately same size. Upper and lower procurrent caudal-fin rays iv-v. All fins with minute odontodes scattered on all spines and rays.
Color in life. Whitish grey to slightly pink, with light grey pigmentation scattered on dorsal surface. Body with five inconspicuous broad saddles of grey pigmentation; first on predorsal plates, second below dorsal fin, third between dorsal and adipose fins, fourth below adipose fin, and fifth on posterior caudal peduncle. Dorsal, adipose and caudal fins with one or two bands of grey spots. Pectoral, pelvic, and anal fins unpigmented. Opercle reddish, due to underlying gill filaments seen by transparency. Some specimens very lightly colored, with faint spots, almost imperceptible. When exposed to continuous illumination in laboratory, fish became slightly darker ( Fig. 1 View Fig ).
Color in alcohol. Generally whitish tan, same pattern as described above but grey pigmentation much fainter ( Fig. 2 View Fig ).
Habitat, distribution and behavior. During our visit in the dry season of 2007, catfish were found throughout the small stream inside the cave Russão III and in the isolated pools of the cave Russão II. Fish were also observed in riffles with moderate water current, but they concentrated in slow moving, soft-bottomed pools with vegetal debris. Other fish were observed in the cave: trichomycterids, Ituglanis (6- 7 specimens observed), and some characids.
Most of the time the catfish remained stationary on the sandy or rocky bottom, occasionally moving over short distances. When foraging on vegetal debris, rocks and crevices, they moved slowly, alone or in pairs, inspecting the substrate with the barbels and mouth. Occasionally, one individual followed the other, touching its rear with the mouth or barbels. No agonistic interactions were noticed. Only when disturbed by the observers they quickly moved away.
In laboratory, the catfish were kept collectively in an 80 l aquarium, in the absence of light (except during maintenance activities and short, random periods of observation) and fed live Artemia crustaceans ad libitum, once or, less frequently, twice a week. These fish are quite tolerant to each other, sharing hiding places (limestone blocks); we did not observe agonistic interactions or other dominance behaviors. They behaved more actively than in the cave habitat, moving not only on the bottom but frequently also in midwater, occasionally surfacing to gulp air, apparently for neutral buoyance maintenance, as observed by Gee & Graham (1987) for many callichthyids. Epigean Aspidoras catfish from São Domingos karst area, also observed in laboratory, were active predominantly near the bottom, using the midwater less frequently than the cave specimens. The latter are very agile, jumping over barriers above the water surface and trying to climb water currents in the aquarium. All individuals promptly accepted live Artemia offered in laboratory, intensifying the midwater activity on such occasions.
Juveniles of the hypogean population born in laboratory at the beginning of 2009 were noticed when already 15+ mm long, all presenting visible eyes, as observed for all troglobitic vertebrates whose development was studied. One year later ( March 2010), these specimens exhibited individual variability regarding eye development, from those with eyes as developed as in epigean fish ( Fig. 5a View Fig ) to individuals without externally visible eyes ( Fig. 5d View Fig ); intermediate stages were also present ( Fig. 5b,c View Fig ). The individual growth rates are low - after three years in laboratory, the wild caught adults did not grow noticeably.
No circadian component was observed for one out of four studied specimens monitored under constant darkness, indicating an incipient regression in time keeping mechanisms parallel to that observed for eyes and pigmentation ( Trajano et al., 2009).
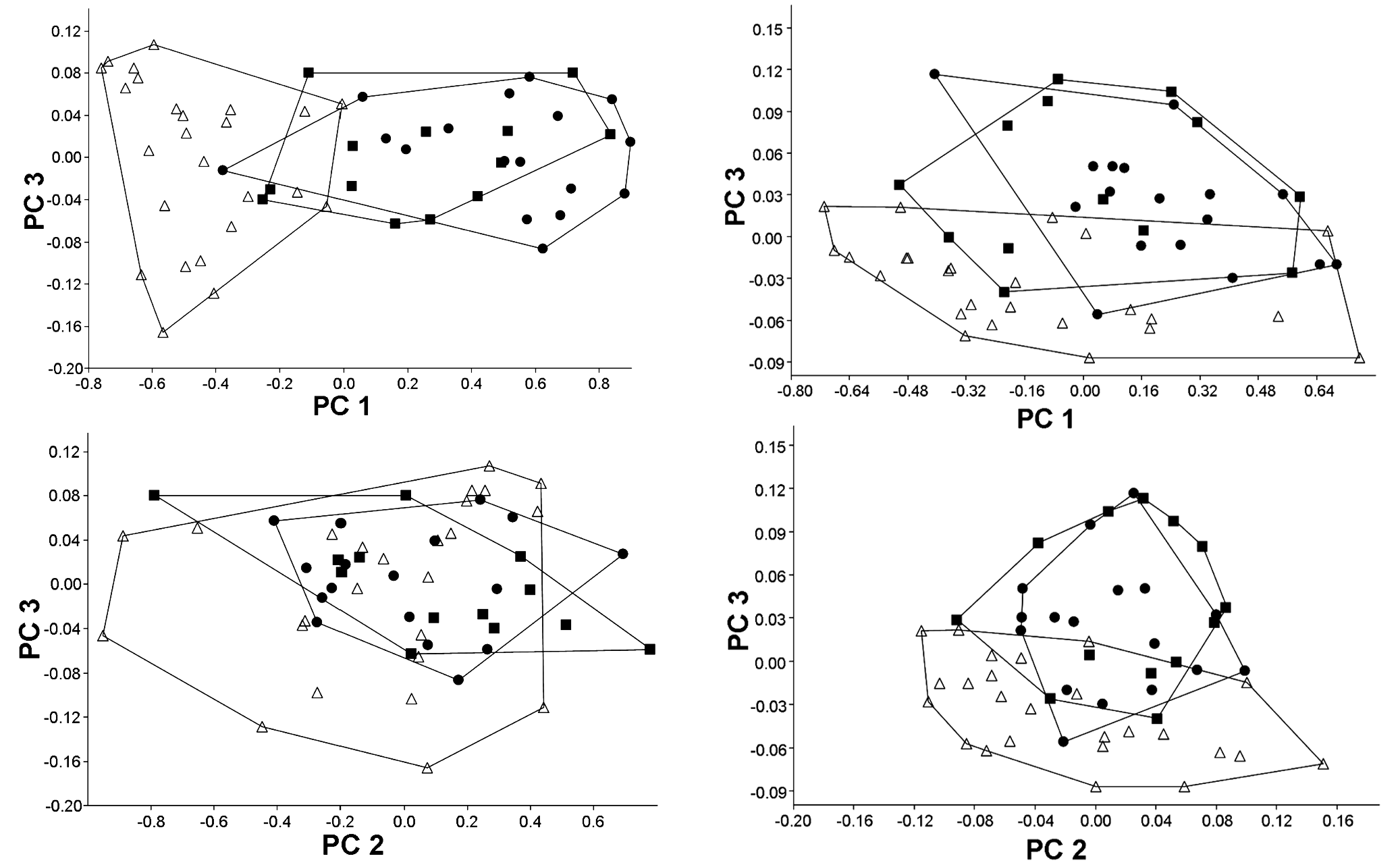
Morphometrics. In a PCA with all measurements the first principal component included 64.9% of the total variance and all variable loadings were positive and varied little in magnitude, except for horizontal orbital diameter, which was strongly negative (loading = -0.861). Plots of factor scores of PC I vs. PC II grouped both cave populations in a broadly overlapping cluster and partially separated the epigean specimens ( Fig. 3). Since the separation is on PC I and the orbital diameter was strongly negative, we believe that it represents a general size factor ( Bookstein, 1989). Plots of factor scores of PC II vs. PC III grouped specimens into three broadly overlapping clusters ( Fig. 3). PC II and III included 31.4 and 0.9% of the total variance, respectively. Measurement with heavier loading on PC II was again the horizontal eye diameter (-0.501), while loadings of all other measurements were around -0.2; heavier loadings on PC III are dorsal spine length (-0.916) and dorsal to adipose distance (0.189).
On a second PCA we removed the three measurements that are directly influenced by the reduction of eyes (horizontal eye diameter, interorbital width, and snout length). In this case, the PC I included a large proportion of the total variance (90.6%) and all variable loadings were positive and varied little in magnitude, suggesting that it represents a general size factor ( Bookstein, 1989). Plots of factor scores of PC I vs. PC II grouped both cave population and the epigean specimens in a broadly overlapping cluster ( Fig. 3). Plots of factor scores of PC II vs. PC III grouped specimens into three broadly overlapping clusters ( Fig. 4 View Fig ). PC II and III included 2.4 and 1.7% of the total variance, respectively. The measurement with heavier loading on PC II was dorsal spine length (0.947), while loadings of all other measurements were negative and below -0.2; heavier loadings on PC III were adipose spine length (-0.560) and dorsal to adipose distance (0.464).
The principal components analysis of morphometric traits failed to discriminate clusters among the two cave populations and among cave and epigean specimens, indicating that morphology not related to eye reduction is highly homogeneous. However, some morphological differences were found between specimens of the epigean populations when compared to specimens from the caves (Table 1). These differences are mainly related to proportions of the head, as head depth, least interorbital distance, horizontal orbital diameter, snout length, and least internareal distance.All these features, however, are probably influenced by modifications of the head morphology caused by the reduction of the eye and orbit, making it difficult to interpret. The taxonomic situation of Aspidoras albater and other potentially undescribed species of Aspidoras in the upper rio Tocantins is in need of attention, and the differences found between the epigean and the cave populations may not be sufficient to delimit a separate species. These differences must be investigated based on a much larger sample and broader geographic representation and using both morphological and molecular evidence.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Aspidoras albater Nijssen & Isbrucker, 1976
| Secutti, Sandro, Reis, Roberto E. & Trajano, Eleonora 2011 |
Aspidoras albater
| Nijssen & Isbrucker 1976: 115 |