Characidium cacah, Zanata & Ribeiro & Araújo-Porto & Pessali & Oliveira-Silva, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4790.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:19472BF2-CFA0-48D7-8856-DFDF32AFFAF8 |
|
persistent identifier |
https://treatment.plazi.org/id/F8BB223F-F0E9-4078-9359-D6FB3DA30963 |
|
taxon LSID |
lsid:zoobank.org:act:F8BB223F-F0E9-4078-9359-D6FB3DA30963 |
|
treatment provided by |
Plazi |
|
scientific name |
Characidium cacah |
| status |
sp. nov. |
Characidium cacah , new species
( Figs. 1–2 View FIGURE 1 View FIGURE 2 )
Characidium View in CoL sp.: Vieira et al., 2005: 81 ( Brazil, Minas Gerais, Serra do Cipó National Park, listed).
Characidium View in CoL sp.: Alves & Pompeu, 2010: 186 ( Brazil, Minas Gerais, rio Cipó, Serra do Cipó National Park, listed).
Holotype. MZUSP 125765 View Materials , 36.5 mm SL, Brazil, Minas Gerais, Jaboticatubas, rio Cipó, Serra do Cipó National Park , rio das Velhas sub-basin, rio São Francisco basin, 19°20’36.2”S 43°36’30.4”W, 783 m a.s.l., 14 May 2019, T. Pessali, F. Porto, I. Penido & S. Santana. GoogleMaps
Paratypes. All from Brazil, Minas Gerais, Jaboticatubas, Serra do Cipó National Park , rio das Velhas sub-basin, rio São Francisco basin : CICMG 93 , 1 , 26.1 mm SL, rio Cipó below meeting of rio Bocaina and rio Mascates , 19°20’49.7”S 43°36’20.5”W, 785 m a.s.l., 25 Sep 2018 GoogleMaps , T. Ribeiro & F. Porto . CICMG 95 , 1 , 26.8 mm SL, rio Bocaina , 19°20’42.1”S 43°36’20.9”W, 789 m a.s.l., 23 Aug 2018 GoogleMaps , T. Ribeiro & F. Porto . CICMG 154 , 3 c&s, 17.9–24.1 mm SL, Lagoa Rasgada , approximately 120 m of the left margin of rio Cipó , 19°20’47.9”S 43°36’40.4”W, 791 m a.s.l., 20 Jun 2019 GoogleMaps , F. Porto & M. Kadri. CICMG 157 , 10 , 20.4 –27.0 mm SL ; MCNIP 4530 , 6 , 21.0– 25.4 mm SL, Lagoa do Boi , approximately 130 m from left margin of rio Cipó , 19°20’53.9”S 43°36’29.6”W, 803 m a.s.l., 15 May 2019 GoogleMaps , T. Pessali, F. Porto, I. Penido & S. Santana . CICMG 158 , 8 , 25.3–36.2 mm SL ; MCNIP 4528 , 2 , 29.0– 36.8 mm SL ; MZUSP 125766 View Materials , 3 View Materials , 27.9–36.2 mm SL ; UFBA 9056 , 8 , 25.3–36.2 mm SL, 1 c&s, 35.5 mm SL ; ZUEC 17227 View Materials , 4 View Materials , 25.6–38.6 mm SL, 1 c&s, 27.5 mm SL, collected with holotype GoogleMaps .
Diagnosis. Characidium cacah can be distinguished from all congeners, except C. chicoi Graça, Ota & Domingues , C. helmeri Zanata, Sarmento-Soares & Martins-Pinheiro , C. mirim Netto-Ferreira, Birindelli & Buckup , C. nana Mendonça & Netto-Ferreira , C. nupelia Graça, Pavanelli & Buckup , C. stigmosum Melo & Buckup , and C. xavante Graça, Pavanelli & Buckup , by the presence of an incomplete lateral line and the absence of the adipose fin. The new species can be distinguished from C. helmeri and C. stigmosum by having 12 circumpeduncular scales ( vs. 13 or 14), from C. chicoi , C. mirim and C. nana by the presence of a thin, inconspicuous or dashed midlateral dark stripe ( vs. presence of conspicuous dark midlateral stripe) and from C. nana , C. nupelia and C. xavante by the absence of a peduncular blotch ( vs. presence). It can be further distinguished from C. helmeri by having a single series of dentary teeth ( vs. two series of dentary teeth), a well-developed supraorbital ( vs. supraorbital reduced or absent) and isthmus completely covered by scales ( vs. anteriormost portion of isthmus naked). The new species further differs from C. chicoi by the presence of a dark rounded inconspicuous humeral blotch, shaped like an upside-down acute triangle).
Description. Morphometric data of holotype and 24 paratypes in Table 1. Body elongate and moderately compressed. Greatest body depth at vertical through dorsal-fin origin. Dorsal profile convex from upper lip to interorbital area, slightly convex from this point to dorsal-fin origin, convex and posteroventrally inclined along dorsal-fin base, almost straight between dorsal-fin terminus to anteriormost caudal-fin procurrent ray. Ventral profile slightly convex near dentary symphysis, straight or slightly convex from that area to pelvic-fin origin, straight from latter point to anal-fin origin, slightly concave along anal-fin base, and straight from anal-fin terminus to anteriormost ventral caudal-fin procurrent ray. Snout triangular in lateral view, rounded in dorsal view.
Mouth subterminal, aligned or slightly lower than ventral margin of orbit. Distal tip of maxilla not reaching vertical through anterior margin of orbit. Orbit approximately round, larger than snout length. Cheek thin, depth approximately a fourth to a fifth of orbit diameter. Nares separated, without distinctly raised margins; posterior naris considerably closer to orbit than to anterior naris. Supraorbital well developed, inner border convex and outer border slightly concave. Nasal bones restricted to the ossified canal. Parietal fontanel limited anteriorly by frontals. Parietal branch of supraorbital canal absent. Dentary teeth in single row with 8 (1), 9 (4) or 10 (3) uni- or bicuspid teeth; teeth decreasing in size from symphysis. Premaxilla with single row of 6 (1), 7* (18) or 8 (6) unicuspid triangular teeth, decreasing in size from symphysis. Maxillary edentulous. Ectopterygoid with a patch of about 6–10 (2) teeth. Mesopterygoid teeth absent. Branchiostegal rays 4 (2), 3 connected to anterior ceratohyal, 1 connected to the posterior ceratohyal.
Scales cycloid; circuli absent on exposed portion of scales; up to 12 divergent radii present on exposed portion of scales. Lateral line reduced; perforated scales 6 (1), 7* (17), or 8 (7); total scales along lateral line series 34 (14) or 35* (11). Horizontal scale rows above lateral line 4* (24) or 5 (1); horizontal scale rows below lateral line 5* (22) or 6 (3). Predorsal scales 9 (1), 10* (8), 11 (10) or irregularly arranged (6). Scale rows around caudal peduncle 12 (25). Isthmus completely covered with scales. Pseudotympanum present as a muscular hiatus at vertical through anterior portion of swimbladder and mostly situated between ribs of the fifth and sixth vertebrae, with large opening anterior to the sixth vertebrae; rib of fifth vertebrae largely evident and a small part of sixth evident ( Fig. 2 View FIGURE 2 ).
Dorsal-fin rays ii,8 (1), ii,9* (23) or iii,9 (1); distal margin of dorsal fin rounded. Adipose fin absent. Pectoral-fin rays 10–11 total rays; unbranched rays ii (1), iii (19) or iv* (5), and posterior total rays 5 (1), 6* (7), 7 (16) or 8 (1) branched rays; first and second branched pectoral-fin rays usually longest; posterior tip of pectoral fin reaching pelvicfin origin in specimens up to 30.0 mm SL and larger specimens with pectoral-fin tip falling short of pelvic-fin insertion. Pelvic-fin rays i,6,i* (14) or i,7 (11); second and third branched rays longest; posterior tip of pelvic fin falling short of anal-fin origin.Anal-fin rays iii,5 (1) or iii,6* (24); posterior margin of anal fin slightly rounded, with second branched ray usually longest; last ray adnate* (24) or simple (1). Caudal-fin rays i,8,8,i* (22), i,8,7,i (2) or i,9,8,i (1). Dorsal procurrent caudal-fin rays 8 (2); ventral procurrent caudal-fin rays 6 (2). Total vertebrae 33 (2); precaudal vertebrae 18 (2); caudal vertebrae 15 (2). Supraneural bones 5 (1) or 6 (1). Epural bones 2 (2). Uroneural bone 1 (2).
Color in alcohol. Ground color of head and body pale brown ( Fig. 1a, 1 View FIGURE 1 c-f). Head with tiny melanophores distributed over dorsal, lateral and ventral surfaces, not forming blotches; dorsum darker than lateral surface and ventral surface clearest. Concentration of melanophores forming dark area from tip of snout to eye, not forming a conspicuous dark stripe. Opercle usually darker than surrounding areas, with higher concentration of melanophores on its central portion or dorsal half. Humeral region with round humeral blotch, variably marked. Midlateral pig- mentation forming a continuous narrow dark stripe in some specimens or a series of dark dashes from area posterior to the humeral blotch to caudal peduncle in other specimens, but not reaching end of caudal peduncle. Scales on flanks with dark borders, forming an overall reticulate pattern; pattern more conspicuous on dorsal half of body. Most specimens with up to 14 somewhat zigzag-shaped narrow bars and less pigmented specimens almost without dark spots or vertical bars. Variation in pattern of bars apparently not related to ontogeny. When present, bars usually reaching at least the horizontal through pectoral-fin origin ventrally; bars located posteriorly to the dorsal fin connected dorsally in some specimens. Ventral portion of the body less pigmented, with sparse tiny melanophores, not forming reticulate pattern or spots. Basicaudal spot variably marked, well visible in some specimens and inconspicuous or absent in others. All fins with melanophores concentrated over rays, interradial membranes mostly hyaline. Proximal portion of dorsal-fin rays and interradial membranes dark, forming a distinctly dark basal area; rays with dark dashes sparsely distributed, usually not forming bands. Caudal fin with dark dashes on rays, usually not forming bands; some specimens with dashes roughly arranged in up to three vertical bands crossing fin. Paired fins and anal fin slightly less pigmented, without dark dashes.
Color in life. Ground color varying from pale yellow to pale brown, slightly olivaceous dorsally and white ventrally ( Fig. 1b View FIGURE 1 ). Parts of iris, infraorbitals, and opercle silvery. Fins pale yellow. Dark marks and spots on body and fins similar to the pattern of preserved specimens.
Etymology. Characidium cacah is named in honor of the ichthyologist Carlos B. M. Alves (Projeto Manuel-zão—UFMG), known to his friends as “Cacá”, for his great contribution to the knowledge of the ichthyofauna of the rio das Velhas basin and for being one of the collectors of the first specimens of the species described herein. A noun in apposition.
Sexual dimorphism. Dissected specimens were not mature sexually and no sexually dimorphic traits were observed.
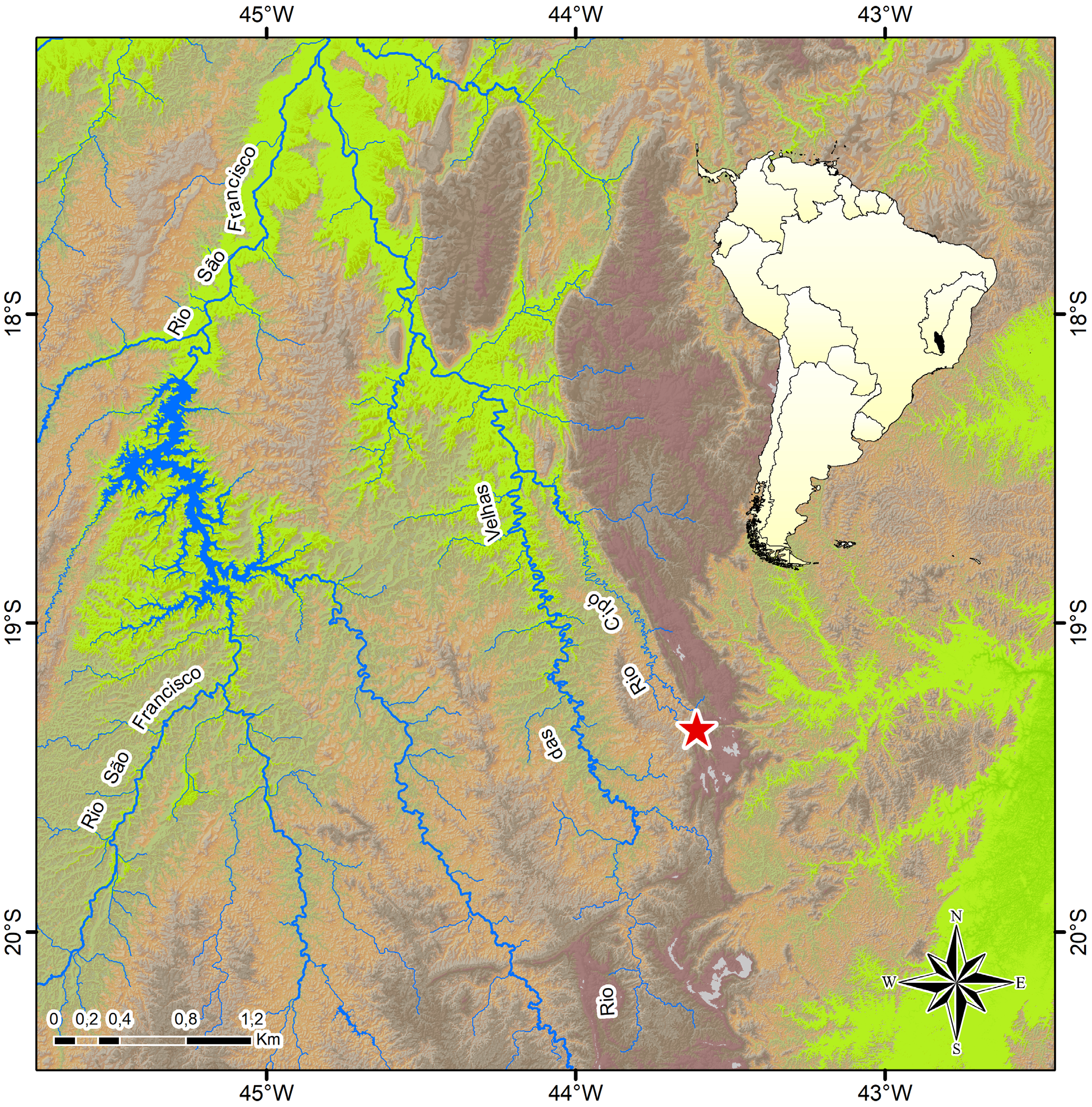
Distribution. Characidium cacah is only known to occur in the rio Cipó drainage, a tributary of the middle rio das Velhas sub-basin, rio São Francisco basin, Minas Gerais State, Brazil ( Figs. 3–4 View FIGURE 3 View FIGURE 4 ).
Habitat and ecological notes. The rio Cipó is formed by the meeting of the rio Mascates and rio Bocaina, which have their headsprings within the Serra do Cipó National Park, possessing several temporary and permanent marginal lagoons mainly located at lower altitudes ( Fig. 4a View FIGURE 4 ). Specimens of C. cacah were caught near submerse macrophytes and marginal grasses in lotic and lentic environments of the rio Cipó sub-basin, at relatively high elevations (783–803 meters a.s.l.). The stretches of the rio Cipó ( Fig. 4 View FIGURE 4 b-c) and rio Bocaina sampled vary from 9–15 meters wide, up to 1 m deep, possessing sandy substrate, riparian forest and slow to moderate water flow, and transparent water with a brown tinge due to the presence of humic and fulvic acids. Syntopic species in lotic sites include Astyanax fasciatus (Cuvier) , A. lacustris (Lütken) , Astyanax sp., Australoheros mattosi Ottoni , Brycon nattereri Günther , Characidium cf. lagosantense, Gymnotus cf. carapo Linnaeus, Hasemania sp., Hemigrammus sp., Hoplias intermedius (Günther) , Knodus sp., Parotocinclus robustus Lehmann A. & Reis , Phalloceros uai Lucinda , and Piabina argentea Reinhardt. Characidium cacah was also sampled in the Lagoa do Boi ( Fig. 4d View FIGURE 4 ) and Lagoa Rasgada, the former a natural lagoon and the latter a man-rectified lagoon with a flooded swampy area. Both possess dark water, muddy bottom and are surrounded by cattle pastures and marginal macrophytes. In these relatively shallow (0.2–1.0 m deep) lentic sites, only small juveniles of C. cacah were caught ( 17.9–27.9 mm SL), whereas the larger specimens were sampled only in the lotic sites. In the lagoons, C. cacah was sampled syntopically with A. lacustris , A. mattosi , C. cf. lagosantense , Hasemania sp., H. intermedius , and P. uai .
Conservation status. Characidium cacah had been collected in five nearby localities in the rio Cipó drainage, rio das Velhas sub-basin. Several anthropogenic impacts are reported for the rio das Velhas sub-basin, among them pollution by sewage effluents and siltation ( e.g., Alves & Pompeu, 2005; Pompeu et al., 2005; Leal et al., 2011; Ferreira et al., 2012). However, C. cacah occurs within the Serra do Cipó National Park, a conservation unit. According to Pompeu et al. (2005) and Carvalho et al. (2019), the rio Cipó is one of the best-preserved major tributaries of the rio das Velhas watershed. We thus consider that C. cacah could be classified as Least Concern (LC), according to the International Union for Conservation of Nature Standards and Petitions Committee (IUCN, 2019).
Remarks. Characidium cacah shares the absence of the parietal branch of the supraorbital canal, used by Buckup (1993b) to diagnose the Clade C4 group of species that includes C. bahiense , C. interruptum Pellegrin and C. lanei Travassos. More recently, the taxonomic limits of Clade C4 were expanded with the addition of C. lagosantense , C. laterale (Boulenger) , C. mirim , C. nupelia , C. occidentale Buckup & Reis , C. orientale Buckup & Reis , C. rachovii Regan , C. stigmosum , C. vestigipinne Buckup & Hahn , and C. xavante , and the putative monophyly of the clade supported also by the presence of more than 12 bars on body and the presence of a single row of dentary teeth ( Netto-Ferreira et al., 2013). However, according to Melo & Espíndola (2016), these characters were not evaluated correctly in some of the species included in the group and are not useful to diagnose the clade C4 as recently proposed. At any rate, C. cacah shares the three features proposed up to date to circumscribe the Clade C4 and other reductive characters with subgroups of the cited species. The new species shares an incomplete lateral line, the absence of the adipose fin, and the absence of the parietal branch of the supraorbital canal exclusively with C. chicoi , C. nana , C. nupelia , C. stigmosum , and C. xavante , and shares the presence of only four branchiostegal rays with C. bahiense , C. nana , C. nupelia , C. stigmosum , C. summus Zanata & Ohara and C. xavante . Characidium cacah also shares with species from the C4 clade and some other congeners an overall shape of body and fins related to species dwelling in slow to moderate water current, and does not possess adaptations of congeners that inhabit fast water current environments, as a streamlined body and paired-fins modifications, as discussed by several authors ( e.g., Buckup et al., 2000; Zanata & Ohara, 2015; Zanata et al., 2015; Zanata et al., 2018). A comprehensive phylogenetic study of Characidium is necessary to properly evaluate the evolution of the morphological traits shared by C. cacah with congeners and to define the relationships of the species.
Comparative material examined (additional to Zanata et al., 2018): Characidium mirim : MZUSP 111123 ( holotype, 20.2 mm SL), Brazil, Mato Grosso, rio das Mortes. Characidium satoi : MZUSP 115059 View Materials ( paratypes, 17, 26.2–44.3 mm SL), Brazil, Minas Gerais, Córrego Curral das Éguas, tributary of rio Abaeté, rio São Francisco basin. Characidium tapuia : UFBA 8511 ( paratypes, 3, 29.8–32.1 mm SL, 1 c&s, 30.8 mm SL), Brazil, Maranhão, rio Balsas basin .
| T |
Tavera, Department of Geology and Geophysics |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Characidium cacah
| Zanata, Angela M., Ribeiro, Thiago C., Araújo-Porto, Felipe A., Pessali, Tiago C. & Oliveira-Silva, Leonardo 2020 |
Characidium
| Alves, C. B. M. & Pompeu, P. S. 2010: 186 |
Characidium
| Vieira, F. & Santos, G. B. & Alves, C. B. M. 2005: 81 |