Alox chaunos Galil & Ng, 2007
|
publication ID |
https://doi.org/10.11646/zootaxa.4111.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:81E1AE11-FCD1-4AED-9C7B-155D2C2BC246 |
|
DOI |
https://doi.org/10.5281/zenodo.6078115 |
|
persistent identifier |
https://treatment.plazi.org/id/03FDEE59-FF9C-A761-FF72-637B01AAFBD2 |
|
treatment provided by |
Plazi |
|
scientific name |
Alox chaunos Galil & Ng, 2007 |
| status |
|
Alox chaunos Galil & Ng, 2007 View in CoL
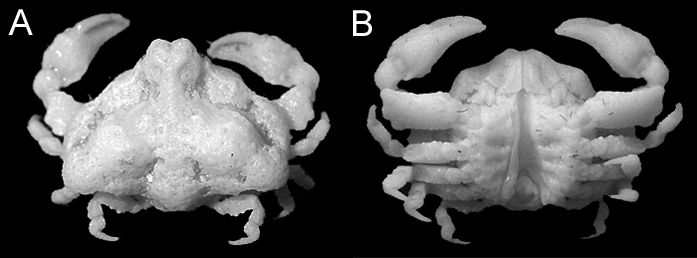
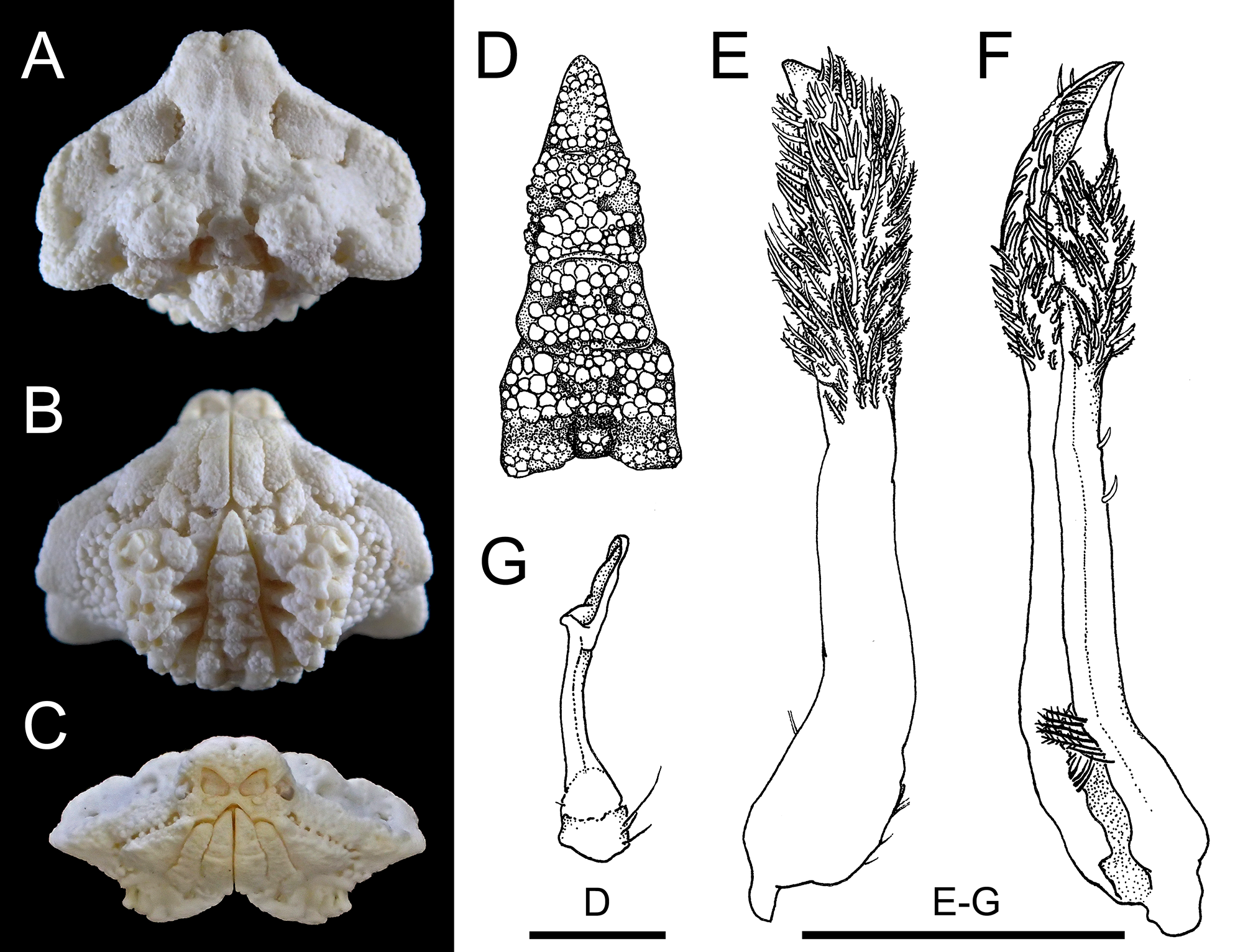
[New Japanese name: Fuka-mizo-karuishi-kobushi] ( Figs. 1–4 View FIGURE 1 View FIGURE 3 View FIGURE 4 )
Alox View in CoL somphos— Marumura & Kosaka, 2003: 26 [not A. somphos Tan & Ng, 1995 View in CoL ]
Alox chaunos Galil & Ng, 2007: 80 View in CoL –81, figs. 1B, 5H, I [ type locality: Momo Beach, Panglao, Philippines].
Material examined. Holotype: NMCR No. 29900, male (CL 6.8 mm), station M7, Mono Beach, Panglao, 09°36.1’N 123°45.2’E, 0–3 m, reef platform with seagrass, 1 June 2004 (photographs).
Additional material: WMNH-Na-Cr 0 163, 1 female (8.9 × 12.1 mm), Nakagusuku Bay, Okinawa Island, 9 April 1993, coll. S. Nagai (examined by Marumura & Kosaka 2003).—RUMF-ZC-2942, 1 male (6.6 × 8.6 mm), 2 females (11.3 × 15.4, 10.6 × 14.1 mm), Minatogawa, Urasoe City, Okinawa I., 9 September 2010, coll. T. Maenosono.—RUMF-ZC-2946, 1 male (5.9 × 7.8 mm), same locality, 22 January 2011, coll. T. Maenosono.—RUMF-ZC-2943, 1 male (5.9 × 7.5 mm), Kaichu Doro, Uruma City, Okinawa I., 4 March 2011, coll. T. Maenosono.—RUMF-ZC-2945, 3 females (6.2 × 7.9–9.4 × 12.7 mm), same locality, 13 December 2008, coll. T. Maenosono.—RUMF-ZC-2944, 1 male (8.1 × 10.3 mm), Awase, Okinawa City, Okinawa I., 15 March 2010, coll. T. Maenosono.—NSMT-Cr 24069, 1 male (8.2 × 11.0 mm), 1 ovigerous female (8.1 × 11.2 mm), Nagura Bay, Ishigaki I., 0.5 m, coral gravels, 5 July 2011, by hand, coll. N. Ohtsuchi.—CBM-ZC 13077, 1 male (7.8 × 9.9 mm), 1 female (7.4 × 9.8 mm), same locality, 10 April 2013, by hand, coll. K. Nakamoto.—RUMF-ZC- 3745, 2 females (9.4 × 12.6, 9.2 × 12.4 mm), same data.—NSMT-Cr 24070, 3 females (7.6 × 10.0–8.6 × 11.0 mm), 11 April 2013, same locality, by hand, coll. K. Nakamoto.—RUMF-ZC-3746, 1 male (3.1 × 3.8 mm), same locality, 23 July 2013, by hand, coll. K. Nakamoto.—NSMT-Cr 24071, 1 male (3.3 × 4.4 mm), 1 female (2.3 × 3.0 mm), 1 ovigerous female (9.2 × 12.5 mm), same data.—NSMT-Cr 24072, 1 male (5.3 × 6.8 mm), 1 female (10.9 × 14.1 mm), same locality, 18 October 2013, by hand, coll. K. Nakamoto.—NSMT-Cr 24073, 1 male (7.2 × 8.9 mm), same data.—NSMT-Cr 24074, 1 female (5.5 × 7.3 mm), same data.—CBM-ZC 13078, 1 female (14.3 × 15.6 mm), same data.—NSMT-Cr 24075, 1 male (3.8 × 4.9 mm), 31 October 2013, same locality, by hand, coll. K. Nakamoto.—CBM-ZC 13079, 1 male (10.0 × 13.5 mm), 16 April 2014, same locality, by hand, coll. J. Hayakawa.—CBM-ZC 7080, 1 male (8.3 × 10.8 mm), grass beds, subtidal, Uehara Beach, Iriomote I., Yaeyama Is., 8 July 2001, dip net, coll. T. Komai.
Comparative material. Alox rugosum (Stimpson, 1858) : WMNH-Na-Cr 0 161, 1 female (8.1 × 11.6 mm), Nakagusuku Bay, Okinawa I., 10 m, 27 March 1991, coll. Kubo (dried specimen).—RUMF-ZC-3743, 1 male (6.4 × 9.2 mm), Sunabe, Chatan-cho, Nakagami-gun, Okinawa I., 3 m, 24 May 2014, SCUBA, coll. R. Yoshoda.—RUMF-ZC-3744, 1 female (4.8 × 6.7 mm), same data.—CBM-ZC 3999, 1 male (7.2 × 10.2 mm), coral sand, 15–20 m, south of Philippines, 9 May 1997, dredge, coll. T. Komai.— Alox uru Naruse & Ng, 2006 : CBM-ZC 8906, holotype male (4.4 × 5.8 mm), off Minami-Ukibaru I., Nakagusuku Bay, Ryukyu Is., 26°18.966’N, 127°58.460’E, 3 m, coll. T. Naruse & Y. Matsuno, SCUBA and dredging, 20 December 2005.
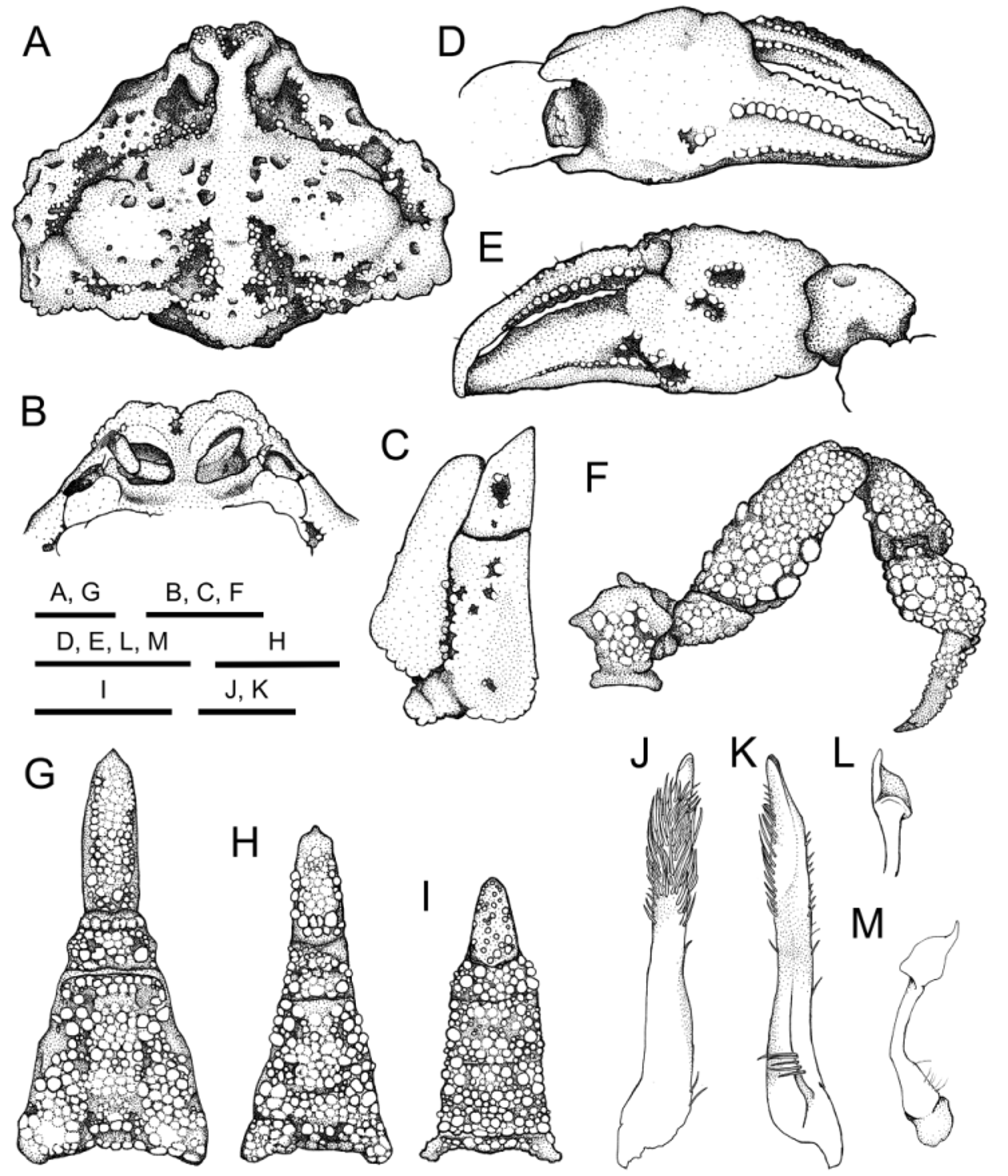
Redescription on basis of specimens from Japan. Carapace (Figs. 2A, D, 3A) subpentagonal, divergent posteriorly, expanded laterally, 1.3 broader than length (mean CL/CW = 1.3±0.0, N = 31); surface densely covered with mushroom-like tubercles, irregularly pitted. Frontal region produced anteriorly, divided into 2 rounded lobes, with elongate cavity apically, upturned in frontal view (Figs. 2C, F, 3A). Postfrontal protuberance wide, sometimes demarcated from median keel (Figs. 2A, D, 3A). Median keel distinct, generally Y-shaped, weakly broadened posteriorly, forming straight longitudinal ridge which reaches midway to cardiac region, not demarcated from cardiac region, with pair of nostril-like postfrontal cavity aside (Figs. 2A, C, 3A). Anterolateral region moderately eroded, with distinct groove on surface; groove parallel to anterolateral carapace borders, deep, granule-lined generally on floor, sigmoid in dorsal view; anterior half continuous to postfrontal cavity, distinctly narrowed near hepatic region; posterior half deep, moderately broad (Figs. 2A, D, 3A). Hepatic region convex due to median protuberance (Fig. 2A, D). Subhepatic margin with low, broad protuberance (triangular facet), barely visible in dorsal view (Figs. 2B, E, 3A). Hepatic, anterolateral margins divided by shallow convcavity. Anterolateral margin slightly sinuous, indistinctly rimmed, separated from posterolateral margin with shallow indentation. Branchiostegal region densely covered with small flattened tubercles (Fig. 2B, E). Branchial regions moderately inflated, with 2 low nodules obliquely from anterior slope to summit, separated from posterior posterolateral margin by narrow or nearly closed groove (Figs. 2A, D, 3A). Posterolateral margin right-angled, rounded apically, nearly straight in anterior half, horizontal, bilobed in posterior half. Cardiac region low, covered with flattened tubercles indistinctly, separated from branchial, intestinal regions by deep, granule-lined groove of variable prominence (Figs. 2A, D, 3A, 4A). Intestinal region moderately elevated, granulate on surface. Posterior margin slightly convex, moderately rounded (Fig. 2A, B).
Basal antennular segment ( Fig. 3 View FIGURE 3 B) opeculiform, occupying antennular fossa when closed; anterior margin of fossa raised, forming rim. Basal antennal segment narrow, occupying hiatus between rim of antennular cavity, orbital submargin. Orbits small, not inflated, lateral margin bisutured. Ocular peduncles sealed by orbit when retracted.
Third maxillipeds ( Fig. 3 View FIGURE 3 C) densely covered with flattened granules; ischium trapezoidal, with blunt ridge diagonally, irregularly pitted mediolaterally; meri subtriangular, with deep median depression (Figs. 2C, F, 3C).
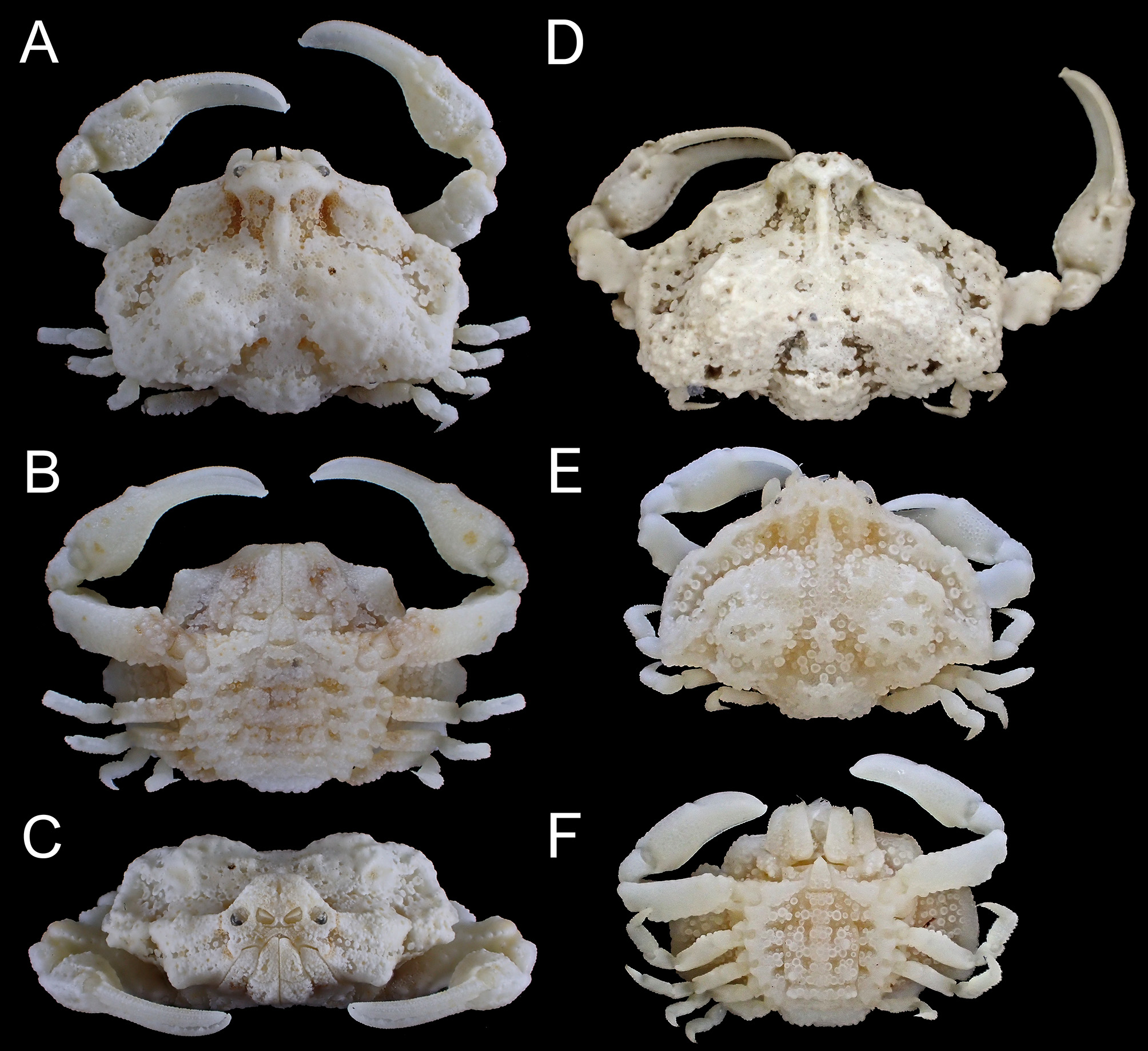
FIGURE. 2. Alox chaunos Galil & Ng, 2007 . Male (A–C, 8.1 × 10.3 mm, RUMF-ZC-2944), Awase, Okinawa City, Okinawa Island, and female (D–F, 8.1 × 11.2 mm, NSMT-Cr 24069), Nagura Bay, Ishigaki Island; G, H, medium-sized specimens of male (G, 5.9 × 7.5 mm, RUMF-ZC-2943) and female (H, 6.2 × 7.9 mm, RUMF-ZC-2945), Kaichu Doro, Uruma City, Okinawa Island. A, D, dorsal view; B, E, G, H, ventral view; C, F, frontal view.
Chelipeds (Figs. 2, 3D, E, 4) stout, subequal in length and shape. Merus trigonal in cross section, densely covered with indistinct flattened tubercles; upper flexor margin irregularly tuberculate in distal half, dilated distally to form rounded lobe; upper extensor margin with 4 or 5 tubercles on distal half, distal two fused at base; lower flexor margin lined with low tubercles; flexor surface with large proximal tubercle, on crossing point of upper, lower flexor margins. Carpus densely granulate. Chela (Figs. 2B, E, 3D, E) stout, scoop-like when both fingers closed; palm eroded proximally, covered with indistinct flattened tubercles, irregularly pitted, distinct median depression on upper surface; dactylus longer than palm (DL/PL = 1.4±0.1, N = 12); immovable finger stout, overlapping dactylus at tip, inner surface with median cavity proximally, outer surface with two parallel transverse rows of small granules in between, upper row reaching half way to tip from proximal end, lower row reaching tip from proximal end, with median cavity proximally, ventrolateral margin with row of relatively distinct granules. Dactylus with broad median keels fringed with relatively distinct granules on both inner, outer surface.
Ambulatory legs (Figs. 2, 3F, 4) short; meri stout, densely covered with flattened granules, with row of large tubercles on proximal half to four-fifths of extensor margin, on upper, lower angle of flexor surface; carpi, propodi, dactyli densely covered with low, sometimes indistinct granules; granules on surface of dactyli, microscopic, subacute.
Male thoracic sternum ( Fig. 3 View FIGURE 3 B) densely covered by mushroom-like tubercles of irregular sizes, moderately sunk medially, horizontal ridges interspaced with 3 deep, granule-lined grooves. In adult females with expanded abdomen, thoracic sternum densely covered by indistinct flattened tubercles on surface ( Figs. 3 View FIGURE 3 E, 4B). Sternoabdominal cavity deep, reaching buccal cavity in both sexes ( Figs. 3 View FIGURE 3 B, E, G, H, 4B).
Male abdomen (Figs. 2B, 3G) hastate, densely covered with mushroom-like tubercles of irregular sizes; first, second somites short; third to fifth somites fused; third, fourth somites eroded medially, elevated laterally; fifth somite eroded laterally, several mushroom-like tubercles on floors of eroded parts; sixth somite trapezoid, with small distinct concavity laterally; telson distinctly narrow, elongate, almost 3 times longer than basal width (TL/ TW = 3.1±0.2, N = 4), 3 times longer than sixth somite (TL/ SSL = 3.1±0.1, N = 4). In females, abdomen prominently domed, densely covered with large, rounded granules on surface, with broad median keel defined by shallow but distinct submedian furrows (Fig. 2E); telson relatively elongate, shorter than in male, with broad, shallow median depression (Figs. 2E, 4B).
G1 ( Fig. 3 View FIGURE 3 J, K) stocky, slightly twisted in distal half, tapering; lateral surface with long setae subdistally; mesial surface without setae; tips obtuse, directing dorsally, without setae. G2 ( Fig. 3 View FIGURE 3 L, M) slender, bent dorsally, one-third length of G1; tips dilated, spoon-like.
Ontogenetic morphological changes. Male telson is elongate, almost 3 times longer than its basal width and sixth somite length (TL/TW = 3.1±0.2, TL/ SSL = 3.1±0.1, N = 4) in larger specimens (CL = 7.2–8.2 mm, Figs. 2C, 3G); in medium-sized specimens (CL = 3.8–6.6 mm), it is less elongate, 2.5 times longer than the basal width (TL/ TW = 2.5±0.0, N = 5, Figs. 2G, 3H), and more than twice the length of the sixth somites (TL/ SSL = 2.1±0.1, N = 5); whereas it is short, 1.5 times longer than its basal width and sixth somite length in smaller ones (CL = 3.1, 3.3 mm, Fig. 3 View FIGURE 3 I). In females, abdomen is prominently domed, and with short telson (TL/TW = 1.7±0.1, TL/ SSL = 0.8±0.1, N = 6, Figs. 2E, 4B) in larger specimens (CL 9.2–11.3 mm); in medium-sized specimens (CL 5.5–7.6 mm), it is flattened, and with elongate telson (TL/TW = 1.8±0.0, TL/ SSL = 1.3±0.0, N = 3, Fig. 2H); whereas it is generally subtriangular with triangular telson (TL/TW = 1.4, TL/ SSL = 1.2, N = 1) in a small specimen (CL 2.3 mm).
Variations. The numerous small pits on the dorsal carapace surface vary in prominence among individuals ( Figs. 1 View FIGURE 1 A, 2A, D, 3A, 4A), but generally, are more coalesced in smaller specimens. Granule-lined grooves separating cardiac, branchial, and intestinal regions also vary in prominence ( Figs. 1 View FIGURE 1 A, 2A, D, 3A, 4A).
Size. The largest male is 8.2 × 11.0 mm; the largest female is 14.3 × 15.6 mm; the smallest ovigerous female is 8.1 × 11.2 mm.
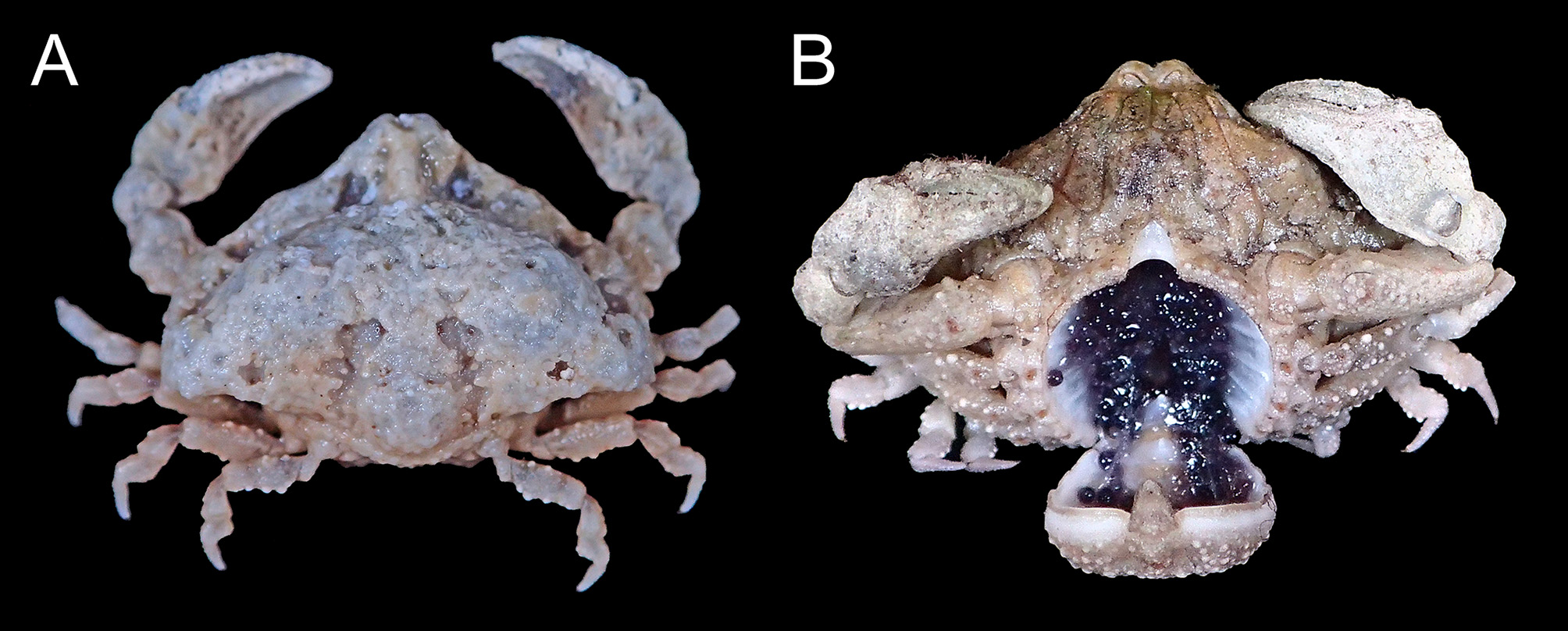
Color in life. Eggs dark purple or reddish brown in fresh samples ( Fig. 4 View FIGURE 4 ) (see also Galil & Ng 2007: 81, figs. 1B).
Distribution. Okinawa, Ishigaki, and Iriomote Islands, Ryukyus, southwestern Japan; Panglao Island, Philippines ( Marumura & Kosaka 2003; Galil & Ng 2007; this study). The present records extend the distribution of this species northwards.
Habitat. Found on reef platforms or sandy bottoms mixed with dead coral gravel in seagrass meadows ( Cymodocea rotundata , C. serrulata , and Thalassia hemprichii in Ishigaki Island), or under dead corals at 0–3 m deep ( Galil & Ng 2007; this study).
Remarks. Male specimens from the Ryukyu Islands agree well with the original description and figures ( Galil & Ng 2007) and the re-examined holotype of Alox chaunos ( Fig. 1 View FIGURE 1 : NMCR No. 29900). The male abdomen with the elongate telson in the specimens at hand are very similar to the shape of the sterno-abdominal cavity of the holotype male from Panglao, Philippines ( Fig. 1 View FIGURE 1 B), warranting our treating them conspecific. With the series of adult males on hand, we can confirm Galil & Ng’s (2007) observation that the elongate male telson distinguishes A. chaunos from all other congeners for which the male telson had been described or illustrated (cf. Tan & Ng 1995; Naruse & Ng 2006; Galil & Ng 2007, 2009). However, the proportions of the telson do change ontogenetically as noted above, and cannot be used as a distinguishing character when specimens are subadult or small. Other distinguishing characters of A. chaunos from congeners are discussed below.
According to Galil & Ng (2007), Alox chaunos is close to A. rugosum and A. uru . Alox chaunos and A. rugosum share the moderately broad (CW/CL = 1.3), well-eroded carapace with indistinct frontal, anterolateral, and posterolateral rims, the deep cavity on the frontal summit, the distinct frontal and median keels forming Yshaped ridge, and the ambulatory legs bearing tubercles on flexor margin. However, these two species can be distinguished by the following characters: (1) carapace is divergent posteriorly in the large specimen of A. chaunos (Fig. 2A, D) but it is divergent anteriorly only in large specimens of A. rugosum ( Fig. 5 View FIGURE 5 A, cf. Tan & Ng 1995: pls. 6D–F, 7); (2) grooves parallel to anterolateral carapace margins are deep in A. chaunos (Figs. 2A, 3A) whereas in A. rugosum , they are shallow in anterior half, and deep but nearly occupied by mushroom-like tubercles in posterior half ( Fig. 5 View FIGURE 5 A, D, E); (3) anterior posterolateral margins are unarmed in A. chaunos (Figs. 2A, D, 3A), whereas they bear three triangular teeth on the anterolateral angle ( Fig. 5 View FIGURE 5 A, D, cf. Tan & Ng 1995: fig. 12B); (4) subhepatic protuberance is broad, apically obtuse in A. chaunos (Fig. 2B, E, C, G), while in A. rugosum , it is narrow, acuminate and produced downwards (cf. Fig. 5 View FIGURE 5 B, C, F; Tan & Ng 1995: fig. 12B); (5) immovable finger is distinctly broader than the movable finger even in large specimens of A. chaunos (PL/ChH 1.4±0.1, Figs. 3 View FIGURE 3 D, 4B), but both fingers are slender in large specimens of A. rugosum (PL/ChH 1.6–1.7, Fig. 5 View FIGURE 5 A, D, E; Tan & Ng 1995: fig. 12C, pls. 6D–F, 7). This character should be used with caution, however, because the proportional length of fingers change ontogenetically in the latter species ( Fig. 5 View FIGURE 5 D, E); (6) G1 is relatively stouter, shorter, and more tapering in A. chaunos ( Fig. 3 View FIGURE 3 J, K) than in A. rugosum (cf. Tan & Ng 1995: fig. 12I); and (7) distal part of the G2 is spoon-like in A. chaunos ( Fig. 3 View FIGURE 3 L, M) whereas it is elongated and petaloid in A. rugosum (cf. Tan & Ng 1995: fig. 12J). The relatively more eroded lateral carapace region and more strongly produced intestinal region, cited as differences by Galil & Ng (2007), is not noticeable or too variable to be useful, and cannot effectively distinguish A. chaunos (Figs. 2A, 3A, D, 5A) from A. rugosum ( Fig. 6 View FIGURE 6 ).
Alox chaunos View in CoL and A. uru View in CoL share the following characters: the posteriorly divergent carapace with indistinct anterolateral rims, and the broad, non-demarcated median keels forming Y-shaped ridge, the distinct median depression on the ischium of the third maxillipeds, and the scoop-like chelae with stout, immovable fingers. The following characters nevertheless distinguish them: (1) hepatic regions are more produced in A. chaunos View in CoL (Figs. 2A, D, 3A) than in A. uru View in CoL (cf. Naruse & Ng 2006: fig. 2b); (2) ischium of the third maxilliped has a distinct median depression on its surface in A. chaunos View in CoL (Figs. 2B, E, 3C) but this is absent in A. uru View in CoL (cf. Naruse & Ng 2006: fig. 2b); (3) merus of the ambulatory leg has a tuberculate posterior margin in A. chaunos View in CoL ( Figs. 1 View FIGURE 1 , 2, 3F, 4, cf. Galil & Ng 2007: fig. 1B) while in A. uru View in CoL , they are densely covered with granules on upper surface (cf. Naruse & Ng 2006: figs. 1b, 2e); and (4) G1 is slender, tapering in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 J, K, cf. Galil & Ng 2007: fig. 5H, I) whereas it is subdistally dilated in A. uru View in CoL (cf. Naruse & Ng 2006: fig. 2g, h). Direct comparisons on the basis of the present material shows that less protruded front and lower gastric region cited by Galil & Ng (2007) as distinguishing features cannot distinguish these two species (CW/CL = 1.3±0.0 in A. chaunos View in CoL versus 1.32 in A. uru View in CoL ).
Alox liklik Galil & Ng, 2015 View in CoL , recently described based on an adult female specimen from Papua New Guinea, closely resembles A. chaunos View in CoL . The original description of A. liklik View in CoL cannot effectively differentiate these two species. On the basis of the figures and our material, however, we must note that the following characters can be useful to distinguish them: (1) branchial regions of carapace are strongly elevated roundly, with two blunt oblique ridges in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 D), while it is weakly elevated, with only one of the bifurcated ridges obliquely positioned in A. liklik View in CoL (cf. Galil & Ng 2015: fig. 2C, G); (2) frontal region is entire, with the dorsal surface forming a Y-shaped ridge in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 D), whereas it is medially demarcated and elevated on the anterior part in A. liklik View in CoL (cf. Galil & Ng 2015: fig. 2C, I); (3) protuberance on the subhepatic region is less produced in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 E) than in A. liklik View in CoL (cf. Galil & Ng 2015: fig. 2D); (4) third maxilliped has a distinct median depression in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 F) but this is absent in A. liklik View in CoL (cf. Galil & Ng 2015: figs. 6H, 8C); (5) immovable finger is twice as broad as the movable finger basally, with lower margin moderately concave in A. chaunos View in CoL ( Figs. 3 View FIGURE 3 F, 5B), while in A. liklik View in CoL , the immovable finger is as broad as the movable finger, with the lower margin distinctly concave (cf. Galil & Ng 2015: figs. 6G, 8J); (6) merus of the cheliped is covered with subacute tubercles, more granular in A. chaunos View in CoL ( Figs. 3 View FIGURE 3 E, 5E) than in A. liklik View in CoL (cf. Galil & Ng 2015: fig. 6G, H); and (7) female abdomen is densely covered by subacute tubercles, with a distinct median ridge defined by deep grooves on each side in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 E), whereas the surface is covered by flattened tubercles, with the median ridge defined by shallow grooves on each side in A. liklik View in CoL (cf. Galil & Ng 2015: fig. 6G, H).
Re-examination of a female specimen of Alox somphos View in CoL from Nakagusuku Bay, Okinawa Island, Ryukyus (WMNH-Na-Cr 0163: see Marumura & Kosaka 2003), which was the only record of this species from Japan, revealed that it should be referred to A. chaunos View in CoL instead. Alox chaunos View in CoL is distinguishable from A. somphos View in CoL as follows: (1) carapace is posteriorly divergent, and its frontal region is more produced in A. chaunos View in CoL (CL/CW = 1.3, Fig. 2A, D) than in A. somphos View in CoL (CL/CW = 1.5, cf. Tan & Ng 1995: pl. 8A, D), (2) median keel is broad and fused with the cardiac region in A. chaunos View in CoL (Fig. 2A, B) whereas it is narrow and very distinctly demarcated from the cardiac region in A. somphos View in CoL (cf. Tan & Ng 1995: pl. 8A, D); (3) chelae are stout, with broad immovable finger as long as palm in A. chaunos View in CoL ( Figs. 3 View FIGURE 3 D, 4B), but they are slender, with narrow immovable fingers distinctly longer than palm in A. somphos View in CoL (cf. Tan & Ng 1995: pl. 8A, D); and (4) G1 is straight and bearing setae on the lateral surface at the distal one-third in A. chaunos View in CoL ( Fig. 3 View FIGURE 3 J, K, cf. Galil & Ng 2007: fig. 5H, I) but it is narrowed in the distal one-third, weakly bent dorsally, and bearing setae dorsoventrally in A. somphos View in CoL (cf. Galil & Ng 2007: fig. 5C–G). The distribution range of A. somphos View in CoL otherwise includes Vietnam, Palau, Malacca Strait, Singapore, and Indonesia ( Tan & Ng 1995).
| SSL |
Sammlung Langenhan an der Sektion Geophysik der Karl-Marx-Universitat Lepzig |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
SubFamily |
Ebaliinae |
|
Genus |
Alox chaunos Galil & Ng, 2007
| Ohtsuchi, Naoya & Kawamura, Tomohiko 2016 |
Alox chaunos
| Galil 2007: 80 |
Alox
| Marumura 2003: 26 |