Buyda phthisica (Gerstaecker, 1885)
|
publication ID |
https://doi.org/10.11646/zootaxa.2454.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.10537489 |
|
persistent identifier |
https://treatment.plazi.org/id/03FE87CD-5C4F-FF93-FF38-FA1FFCE8FA48 |
|
treatment provided by |
Felipe |
|
scientific name |
Buyda phthisica (Gerstaecker, 1885) |
| status |
|
Buyda phthisica (Gerstaecker, 1885) View in CoL
( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Mantispa phthisica Gerstaecker, 1885: 35 View in CoL ; Penny 1982a: 221 (cit.); 1982b: 446, Figs. 74–78 (redesc.); Penny & Costa 1983: 646, Figs. 17 (redesc.); Type locality: Brazil, Amazonas. Holotype female (EMAU), not studied.
Entanoneura phthisica View in CoL ; Handschin 1960: 208; Stange 1967: 18 (cat.); Penny 1977: 34 (list.).
Buyda phthisica View in CoL ; Hoffman 2002: 253, Figs. 552, 557, 568, 606; Ohl 2004: 161 (cat.).
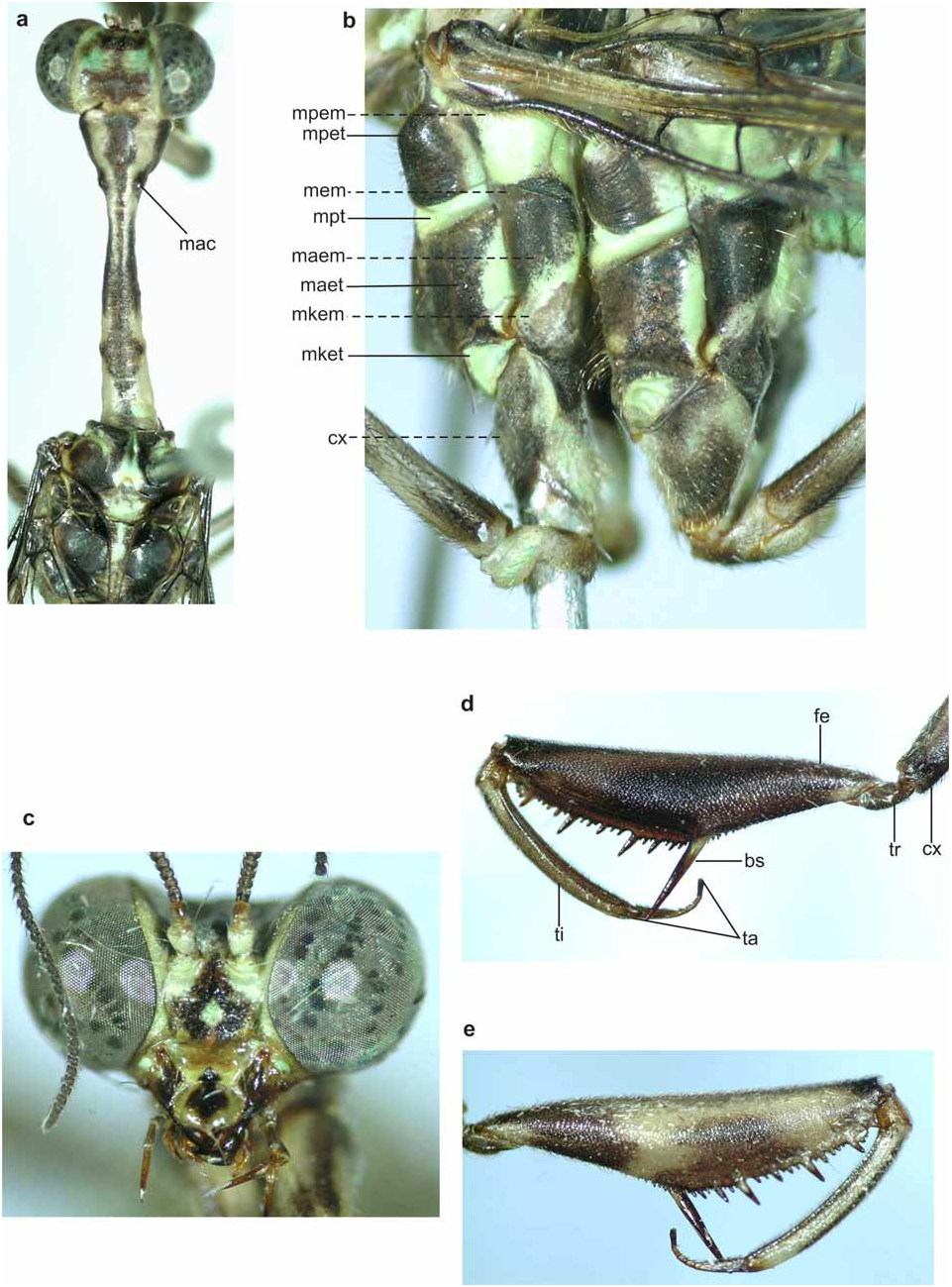
Redescription, male. Vertex green, except for dark brown spots just behind antennae and at posterior region; most specimens with short longitudinal dark brown spot connecting the two spots ( Fig. 2a View FIGURE 2 ). In specimens stored for a long time the spots generally more yellow. Head in frontal view with longitudinal dark-brown stripe beginning between antennae, enlarging at frons and ends at labrum. In some specimens longitudinal head almost entirely brown. Mouthparts reddish-brown with mandibles and apex of palpi slightly dark. Antenna with scape green ventrally and reddish-brown dorsally. Pedicel and flagellum dark brown ( Fig. 2c View FIGURE 2 ).
Pronotum: nearly straight in lateral view, with few setae at proximal and distal regions arising directly from it surface. Length-width-ratio at maculae: 6.1–7.9. Predominantly pale-yellow with large longitudinal dark brown stripe dorsally, wide before maculae and next to the posterior region; two lateral dark brown spots at anterior end, median region and around maculae ( Fig. 2a View FIGURE 2 ). Pteronotum: dark brown except for dorsal green spots next sutures. Some specimens with spots only in central suture ( Fig. 2a View FIGURE 2 ). Scutella dark brown with green central spot, generally large in mesoscutellum ( Fig. 2a View FIGURE 2 ). Scutella with 5–12 pores. Mesopreepimeron, mesepisternum, metapreepimeron and metepisternum green, other pleural sclerites predominantly dark brown with green spots. Mesokatepisternum completely green in some specimens ( Fig. 2b View FIGURE 2 ).
Foreleg: coxa brown except for a large anterior yellowish spot. Trochanter dark brown. Femur posterior surface pale-yellow with three dark brown spots. First beginning near base and extending to basal third; second beginning after the first one and covering almost all region next to spine row, sometimes extending dorsally; third small and placed distally. Spines yellow at base and brown at apex ( Fig. 2e View FIGURE 2 ). Femur anterior surface dark brown except for small yellow spot basally; some specimens with a small yellow spot at base of basal spine ( Fig. 2d View FIGURE 2 ). Tibia pale-yellow except dark brown dorsally. Tarsomere I dark brown except apex as light yellow as other tarsomeres.
Mid and hindlegs: coxae dark brown with three greenish spots ( Fig. 2b View FIGURE 2 ). Trochanters brown at base, green at apex. Femora pale-yellow except base and apex brown. Tibiae generally pale-yellow, sometimes darker. Tarsomeres slightly darker than tibiae. Tarsal claws with four teeth.
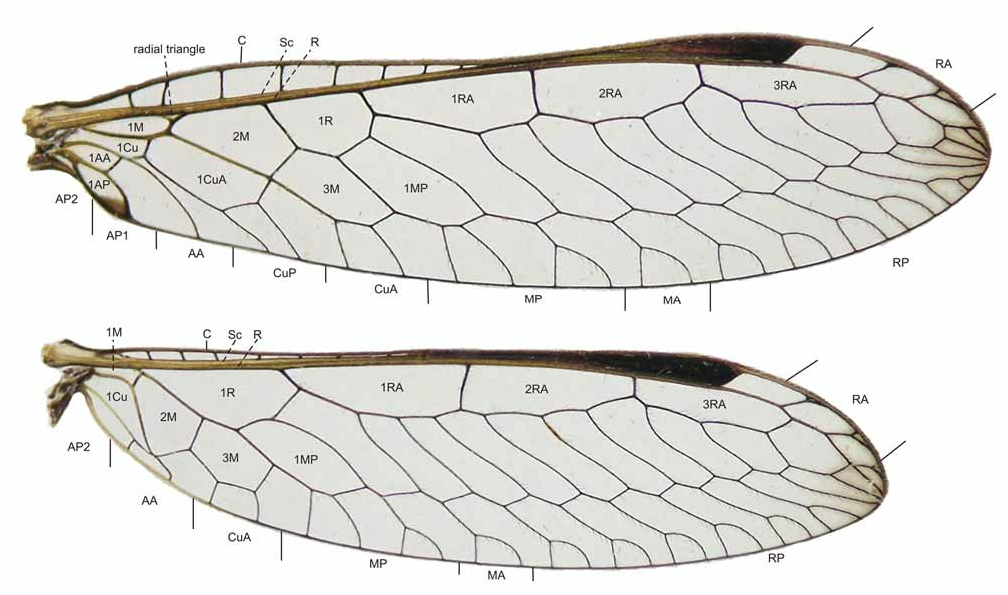
Forewing: length 12.4–18.5 mm, hyaline except apex brown infuscated and space between Sc and RA light brown; 7–9 costal crossveins and 12–17 veins extended posteriorly from RP. Apex of 1AP cell and radial triangle dark brown in some specimens. Pterostigma dark brown. Veins varying between brown and dark brown, except for bases of Sc and RA yellow ( Fig. 3 View FIGURE 3 ). Hindwing: coloration similar to forewing, except vein AP2 and base of AA yellow ( Fig. 3 View FIGURE 3 ); 7–10 costal crossveins and 15–17 veins extended posteriorly from RP.
Abdomen with green to yellow sclerites in specimens stored for long time; central dark brown stripe enlarging posteriorly on tergites and anteriorly on sternites. Pleura dark brown. Specimens darker colored have some sclerites completely dark brown. Pores absent.
Terminalia: ectoprocts extended laterally ( Fig. 4a, d View FIGURE 4 ). Ventromedial lobe apparently separated from remaining ectoproct, with short, thickened setae ( Fig. 4d View FIGURE 4 ). Sternite IX sub-pentagonal, with small flattened apical lobe and 5–7 large setae on each side ( Fig. 4c View FIGURE 4 ). Gonarcus with median lobe wide ( Fig. 4b View FIGURE 4 ). Gonocoxite straight in lateral view with apex rounded, as wide as or slightly wider than mediuncus in ventral view ( Fig. 4f View FIGURE 4 ). Mediuncus wide basally in ventral and lateral view ( Fig. 4e, f View FIGURE 4 ). Pseudopenal membrane shorter than pseudopenis. Hypomere very long and curved ( Fig. 4e, f View FIGURE 4 ) with small rounded tubercles at expanded apex ( Fig. 4g View FIGURE 4 ).
Female. Similar to male except forewing length: 13–19.4 mm, 7–10 costal crossveins, 12–18 veins extended posteriorly from RP; hindwing with 6–10 costal crossveins and 13 to 19 veins extended posteriorly from RP.
Terminalia: Ectoproct larger than gonocoxite ( Fig. 4j View FIGURE 4 ). Sternite VIII large and wide in ventral view, with small median invagination at anterior border in ventral view ( Fig. 4h View FIGURE 4 ). Spermathecal duct coiled, narrowed at base, expanded medially and narrowed again near the fertilization canal; capsule poorly developed ( Fig. 4i View FIGURE 4 ).
Geographical data. Neotropical, with records from Honduras to Uruguay ( Hoffman 2002; Ohl 2004). In Brazil the specimens seem to be more commonly found in the Amazon ( Penny & Costa 1983). Most of the specimens housed in collections are from Central Amazon.
Bionomy. Adults may be collected at any time of year. The immature stages are still unknown, but, however, we obtained eggs and first instar larvae from two female specimens captured in 2005 and kept in laboratory (data not yet published).
Discussion. B. phthsica is easily recognized among other Brazilian species by the color pattern and by the view; e, foreleg, posterior view. Coxa (cx), basal spine (bs), femur (fe), maculae (mac), mesanepimeron (maem), me sa nepist ernum (ma et), mesepi meron (mem), mesokate pime ron (mkem), mesokat epist ernum (mke t), mesopreepimeron (mpem), mesopreepisternum (mpet), mesepisternum (mpt), tarsomeres (ta), tibia (ti), trochanter (tr).
Material examined: Brazil: Amazonas: Manaus, ZF-2, torre 40 m, 02°35’21’’S – 60°06’55’’W, ixii.2004, luz mista ( 28 ♂, 33 ♀ – INPA) GoogleMaps ;. i.1997, ( 1 ♂, 1 ♀ – MZUSP) ; xi.2005, luz móvel ( 1 ♂, 2 ♀ – MNRJ) ; Km 34: Base LBA, 02º35’37’’S – 60º12’39’’W, vii.2008, luz ( 1 ♂ – MPEG) GoogleMaps ; Res [erva] Ducke, iv.1990, ( 1 ♀ – MPEG) ; 02º55’51’’S – 59º58’59’’W, iii.2008, rede entomológica ( 1 ♀ – INPA) GoogleMaps ; Manacapuru, Com [unidade] Lauro Sodré , 03º20’55’’S – 60º37’25’’W, xi.2005, luz ( 1 ♀ – INPA) GoogleMaps ; Manicoré, Cachoeira , 05º29’44’’S – 60º49’21’’W, luz ( 1 ♀ – INPA) GoogleMaps ; Presidente Figueiredo, 02°00’55’’S – 59°49’40’’W, viii.2005, ( 3 ♂, 3 ♀ – INPA); AM 240 , Km 24, x.2008, luz ( 1 ♀ – DZUP) GoogleMaps ; 02º01’05’’S – 59º49’59’’W, luz, ix.2008 ( 1 ♂ – DZUP) GoogleMaps ; Itacoatiara, Madeireira MIL, 02°45’10’’S – 58°39’11’’W, xi.2005, luminosa móvel ( 2 ♂, 2 ♀ –MZUEFS); Rio Abacaxis , 05º15’09’’S – 58º41’52’’W, v.2008, luz sobre o barco, ( 2 ♀ – INPA) GoogleMaps ; Pará: Benevides, Est [rada] Neopolis, Sítio D. Doca, v.1991 ( 1 ♀ – MPEG) ; Rondônia: ix.1966, Mantispa phthisica Gerstaecker det. N.D.Penny, 1982 ( 1 ♀ – INPA) ; Mato Grosso: Nova Mutum: Faz. Buriti, ii.2002 ( 1 ♀ – MZUSP) ; Bahia: Cachoeira Faz. Vila Rial , 14°36’23’’S – 38°53’47’’W, v.2007, luz, ( 1 ♂ – INPA) GoogleMaps .
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
| MPEG |
Museu Paraense Emilio Goeldi |
| DZUP |
Universidade Federal do Parana, Colecao de Entomologia Pe. Jesus Santiago Moure |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Buyda phthisica (Gerstaecker, 1885)
| MACHADO, RENATO JOSÉ PIRES & RAFAEL, JOSÉ ALBERTINO 2010 |
Buyda phthisica
| Ohl, M. 2004: 161 |
| Hoffman, K. M. 2002: 253 |
Entanoneura phthisica
| Penny, N. D. 1977: 34 |
| Stange, L. A. 1967: 18 |
| Handschin, E. 1960: 208 |