Houardiella gracilis Dorchin and Freidberg
|
publication ID |
https://doi.org/10.5281/zenodo.184984 |
|
DOI |
https://doi.org/10.5281/zenodo.6231596 |
|
persistent identifier |
https://treatment.plazi.org/id/03FF87F2-E270-BF5D-FF58-685778B8F804 |
|
treatment provided by |
Plazi |
|
scientific name |
Houardiella gracilis Dorchin and Freidberg |
| status |
sp. nov. |
Houardiella gracilis Dorchin and Freidberg View in CoL , new species
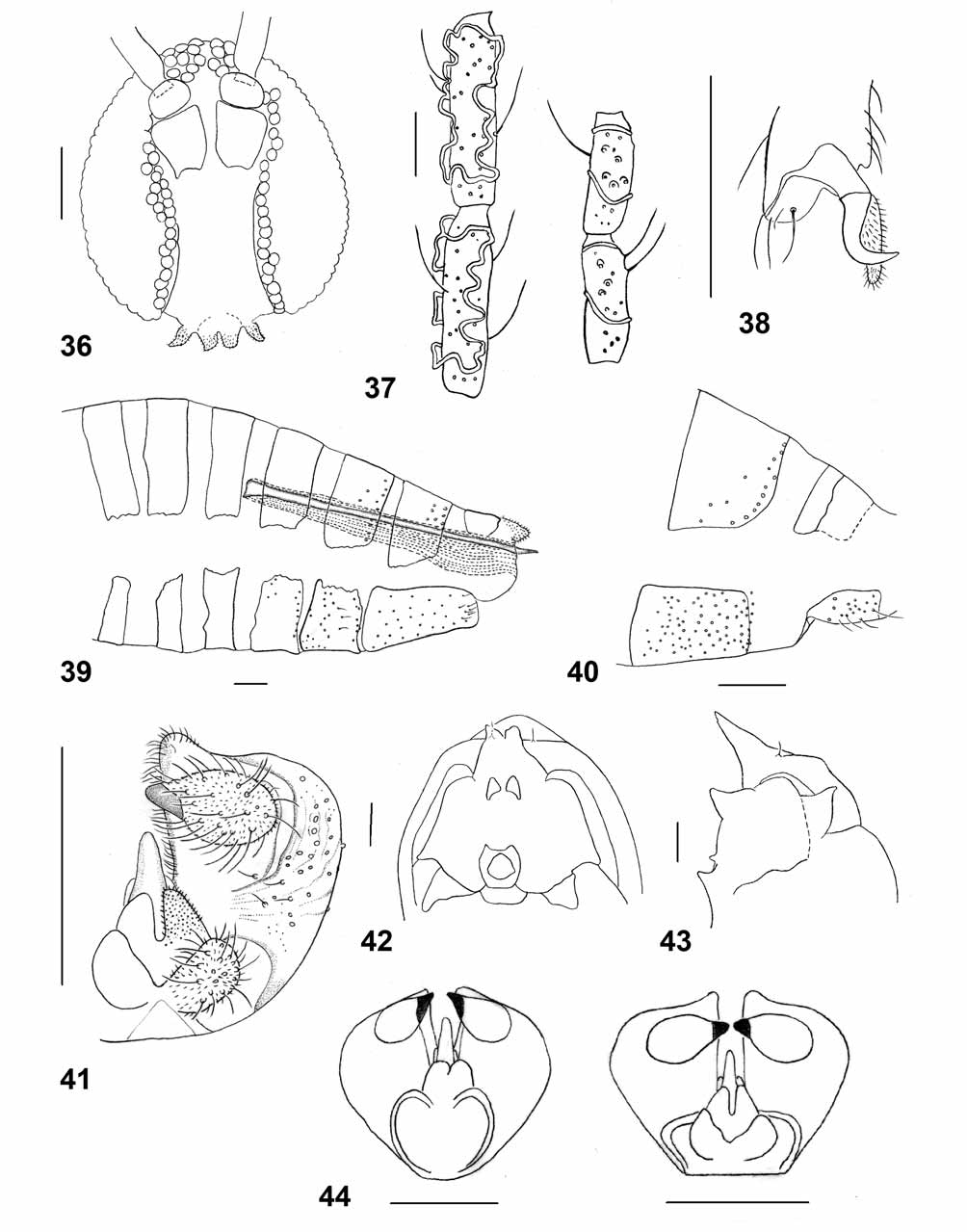
Adult. – Head ( Fig. 36 View FIGURES 36 – 44 ): Eye facets circular, gap between eyes on vertex 0–1 times as wide as facet. Palpus 1- segmented, tapered, setulose. Antenna: cylindrical flagellomeres and sinuous circumfila as in Asphondylia ( Fig. 37 View FIGURES 36 – 44 ).
Thorax: Wing: length 1.6–2.4 mm in females (n=17), 1.6–2.2 mm in males (n=15); transparent; veins Sc and R5 join C at wing mid length, and at wing apex, respectively. Cu forked, very weak. Legs: Claws evenly curved, untoothed on all legs; empodia extend much beyond bend of claw ( Fig. 38 View FIGURES 36 – 44 ).
Female abdomen ( Fig. 39 View FIGURES 36 – 44 ): General color orange, with sparse white hairs. Tergites 2–7 rectangular, with posterior and median rows or groups of setae and evenly distributed scales; trichoid sensilla not detectable. Tergite 8 weakly sclerotized, without strong setae. Sternites 2–6 rectangular, each with posterior row of setae and evenly scattered scales and setae on entire surface. Sternite 7 elongated, with posterior group of stronger setae and evenly scattered scales and setae on entire surface. Ovipositor: Sclerotized part of ovipositor 2.6–3.0 times as long as sternite 7 (n=14).
Male abdomen ( Fig. 40 View FIGURES 36 – 44 ): General color as in female. Tergites 1–7 with posterior row of setae and evenly covered by setulae; tergite 8 sclerotized only proximally, with no or few setae; trichoid sensilla not detectable. Sternites 2–7 with evenly scattered setae on entire surface. Sternite 8 smaller than preceding sternites, with evenly scattered setae. Terminalia ( Fig 41 View FIGURES 36 – 44 ): Gonocoxite robust, widest at distal third, evenly setose ventrally and on sclerotized dorsal part, with dense long setae along median edge, depression around gonostylus, and small posterior projection. Gonostylus teardrop-shaped, with single, entire tooth, evenly setulose, with many evenly scattered long setae. Cerci separated almost to base, evenly setose and setulose. Hypoproct deeply notched, evenly setulose. Paramere smooth, about 0.3 times as long as aedeagus. Aedeagus tapered and apically rounded.
Larva. – Unknown
Pupa. ( Figs. 42–43 View FIGURES 36 – 44 ) – Antennal horns prominent, straight, tapered, divided into two small lobes apically in ventral view, pointed anteriorly. Two well developed facial horns on upper part of eyes, slightly curved anterodorsally. Three lower facial horns arranged in upside-down triangle; median horn much larger than lateral horns, pointed anterodorsally; lateral horns small and pointed. Cephalic seta very short, on slightly elevated base. Dorsum of abdominal segments each with two rows of strong, posteriorly directed barbs.
Holotype – ɗ, Israel, Akko , 12.VIII.2002, N. Dorchin and A. Freidberg, reared from Arthrocnemum macrostachyum stem.
Paratypes – All material from Israel, Akko , reared by N. Dorchin from Arthrocnemum macrostachyum stems, unless otherwise noted. 2Ψ, 1ɗ, same data as holotype; 1Ψ, 1ɗ, 10.II.1995; 1Ψ, 3ɗ, 3.III.1995; 1Ψ, 7.IV.1995; 5Ψ, 20.VII.1996; 1ɗ, 1.VIII.1998; 4Ψ, 2ɗ, 2 exuviae, 20.VII.2002, N. Dorchin & A. Dorchin; 5Ψ, 4ɗ, Deir Hajla, 16.III.2004.
Distribution. – Israel ( Akko salt marsh, Atlit, Northern Dead Sea area).
Etymology. – The species name refers to the relatively small and delicate adults.
Biology. – Larvae develop singly in chambers inside stems of Arthrocnemum macrostachyum . Each chamber occupies a single stem joint that is very slightly inflated and does not differ considerably from normal joints. Infestation was only recognized once adults had emerged, at which point we found emergence holes and exuviae protruding from the stems ( Fig. 8 View FIGURES 5 – 10 ). Adults emerged from plants during spring and summer (February–April and July–August), suggesting that the species completes at least two generations a year.
Remarks. – The genus Houardiella contains two species – H. salicorniae Kieffer from Salicornia fruticosa in Tunisia and Libya ( Kieffer 1912), and H. distincta (Fedotova) from Kalidium schrenkianum in Kazakhstan ( Fedotova 1984). The new species is placed in the genus Houardiella as it shares with its two congeners the single-segmented palpus and the shape of the male terminalia: the gonostylus has a single tapered tooth, and the gonocoxite has a distal projection that extends beyond the gonostylus. Houardiella distincta differs from the other two species in the very narrow base of the male terminalia, which gives them a heart-shaped appearance ( Fig. 44 View FIGURES 36 – 44 ). The length of the sclerotized, needle-like part of the ovipositor relative to sternite 9 of the abdomen is a useful measure for comparison among some Asphondyliini species. This ratio is much smaller in H. distincta (1.36, in the single paratype we examined) relative to that in H. gracilis (at least 2.5). Females of H. salicorniae have not been described. Houardiella salicorniae was described from Salicornia fruticosa , a host that is closely related to A. macrostachyum , and was recorded from a salt-marsh habitat in Tunisia and Libya ( Kieffer 1912, Houard 1922). However, the galls induced by this species differ significantly from those of H. gracilis , constituting scaly, fusiform, and multi-chambered swellings of lateral stems as opposed to the simple, single-chambered and barely conspicuous galls of H. gracilis . Houardiella distincta also induces large and conspicuous galls on its salt-marsh host plant, Kalidium schrenkianum .
In Israel, we reared adults of an undescribed Houardiella species from normal-appearing stems of Anabasis articulata in the northern Dead Sea area. The adults in this population appear to be morphologically indistinguishable from those of H. gracilis , and a genetic study is probably needed in order to determine whether that population represents a different species.
The genus Houardiella may represent a subgroup within Asphondylia that is associated with succulent, articulated Chenopodiaceae in salt-marsh habitats. It is noteworthy that a couple of species ( Asphondylia sarcocorniae and A. floriformis ) recently described in Australia from such a plant ( Sarcocornia quinqueflora ) exhibit morphological attributes that are intermediate between Asphondylia and Houardiella , namely one wide tooth on the gonostylus and a rudimentary third palpal segment (Veenstra-Quah et al. 2007). These two species grouped together in a phylogenetic analysis of chenopod-feeding Asphondylia spp. in Australia ( Kolesik and Veenstra-Quah 2008) and may represent a transitional state between Asphondylia and Houardiella .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |