Stefaniella brevipalpis Kieffer, 1898
|
publication ID |
https://doi.org/10.5281/zenodo.184984 |
|
DOI |
https://doi.org/10.5281/zenodo.6231600 |
|
persistent identifier |
https://treatment.plazi.org/id/03FF87F2-E275-BF40-FF58-6D9278A4FD04 |
|
treatment provided by |
Plazi |
|
scientific name |
Stefaniella brevipalpis Kieffer, 1898 |
| status |
|
Stefaniella brevipalpis Kieffer, 1898 View in CoL
Stefaniella brevipalpis Kieffer, 1898: 56 View in CoL
Neotype of Stefaniella brevipalpis View in CoL designated here – Ψ, Israel, Akko View in CoL , 7.IX.2002, A. Freidberg, reared from Atriplex portulacoides View in CoL stem gall. The neotype is designated in order to clarify the taxonomic status of S. brevipalpis Kieffer. View in CoL Material representing this species is not found among specimens in the USNM or in the Muséum National d’Historire Naturelle in Paris, France (MNHN) (R. Gagné, personal communication). Stefaniella brevipalpis View in CoL was described from an undisclosed locality in Italy, and is the only Stefaniella View in CoL species known from Atriplex portulacoides View in CoL . The galls were later described and illustrated by Houard (1908). The individuals we reared in Israel developed in the same host plant and the same type of galls from which the original name-bearing type had been described. Based on this information and on the original morphological description of the gall midges, we consider our specimens to be adequate for neotype designation. The neotype is deposited in the National Collection of Insects, Zoological Museum, Tel Aviv University, Israel (TAUI). The following description ensures recognition of the designated specimen, and characters differentiating the species from other congeners are detailed below under the remarks section.
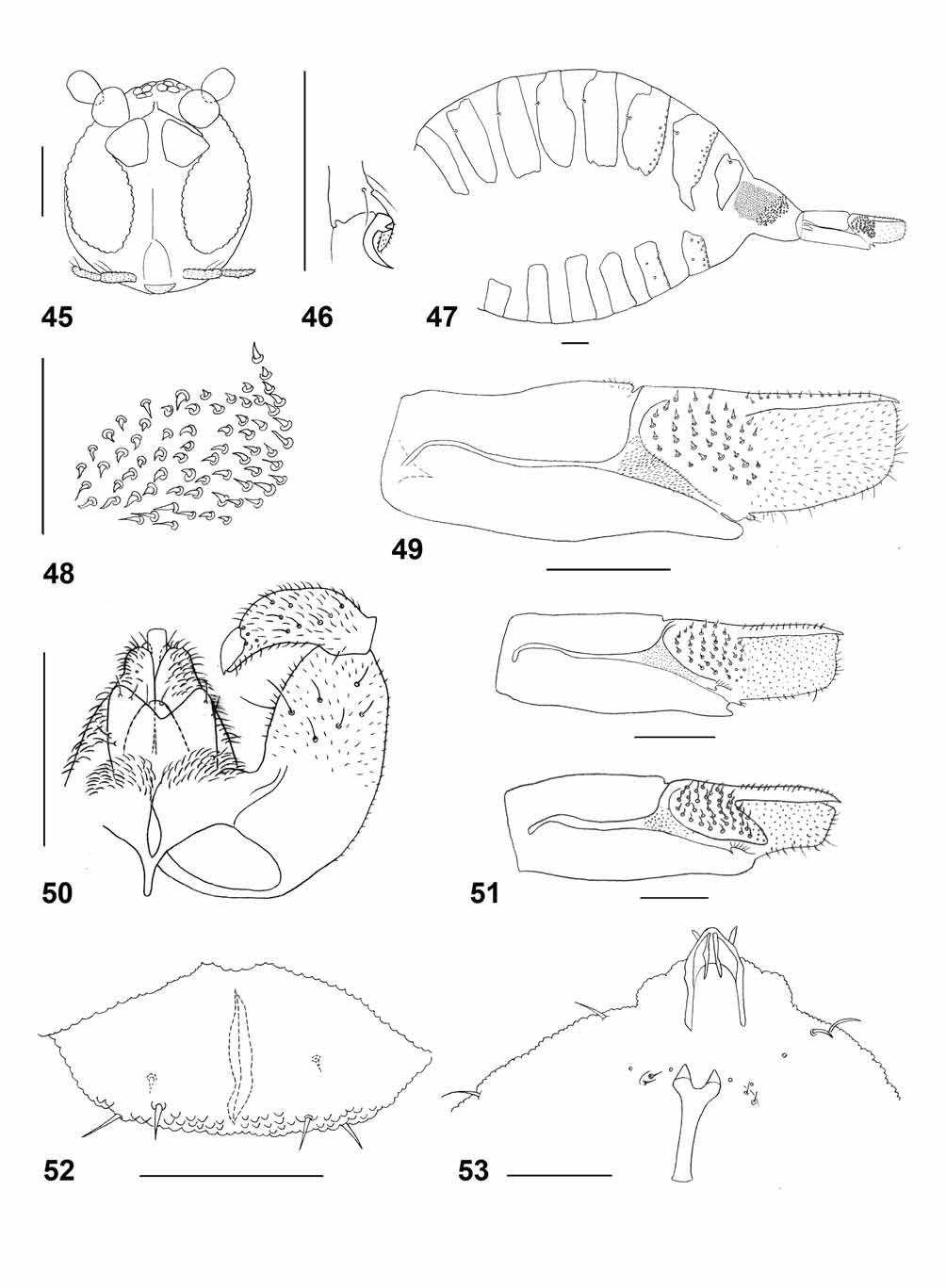
Adult. – Head ( Fig. 45 View FIGURES 45 – 53 ): Eye facets circular, less ordered and farther apart on vertex than laterally, gap between eyes on vertex 0.5–1.5 times as wide as facet. Palpus 2-segmented; second segment somewhat thinner than first, about as long. Antenna similar in both sexes; scape conical, pedicel globular, 9–10 flagellomeres, barrel-shaped, about 1.3 times as long as wide, each with two closely appressed whorls of circumfila, one whorl of setae proximal to basal circumfilum, and group of stronger setae distal to it. Apical flagellomere sometimes comprised of 2–3 fused segments, slightly tapered.
Thorax: Wing: length 1.2–1.4 mm in females (n=5), 1.0– 1.2 mm in males (n=3), transparent. Vein R5 joins C at wing mid length, M present, Cu unforked. C, Sc and R5 with dark scales. Legs ( Fig. 46 View FIGURES 45 – 53 ): Tarsal claws toothed, evenly curved, teeth very short, strongly curved at base. Empodia reaching bend of claw or slightly shorter.
Female abdomen ( Fig 47 View FIGURES 45 – 53 ): Tergites 1–6 rectangular, not extended ventrally, tergite 7 extending ventrally, each tergite with 1–2 posterior rows of setae, anterior trichoid sensilla, and evenly covered by scales; tergite 8 smaller than preceding tergites, tapered ventrally, with anterior trichoid sensilla, without setae or scales. Sternites 2–7 rectangular, with row of posterior setae and several additional, scattered setae; sternite 8 completely undifferentiated from surrounding membrane. Area between tergite 8 and lateral group of setae covered by minute hyaline setae on small bumps. Ovipositor ( Figs. 48–49 View FIGURES 45 – 53 ): Lateral group of setae comprising about 70 relatively short, straight or curved setae. Sclerotized rods widened posteriorly to form weakly sclerotized triangular plate covered by minute spines. Lateral plate sheathing almost entire base of cercus, bearing about 40 straight, short and thick setae that are narrowed abruptly at tip. Aculeus straight and thick, evenly narrowed towards tip, with two rows of minute fine setae; tip of aculeus slightly curved ventrally. Apical lamella rectangular, as long as aculeus, evenly setose.
Male abdomen: Tergites 1–7 rectangular, setation and scales as in female. Tergite 8 completely undifferentiated from surrounding membrane. Sternites 2–7 as in female; sternite 8 shorter and smaller than preceding sternites, without setae or scales. Terminalia ( Fig. 50 View FIGURES 45 – 53 ): Gonocoxite cylindrical, with several strong setae on sclerotized parts, more numerous on ventral part than on dorsal part; mediobasal lobe prominent, evenly setulose. Gonostylus slender, strongly arched, slightly angular at distal third, evenly setulose with several strong setae both dorsally and ventrally. Cerci fused, notched posteriorly, evenly setulose, with several strong setae. Hypoproct entire, distally rounded, evenly setulose, with two strong apical setae. Paramere divided longitudinally into dorsal and ventral lobes; ventral lobe smooth, with apical seta on elevated base; dorsal lobe densely covered by curved setae. Aedeagus slender, truncated at tip, slightly longer than paramere.
Larva ( Figs. 52–53 View FIGURES 45 – 53 ) – White to pale yellow; cylindrical; length: 1.9–2.2 mm (n=4). Integument bumpy on entire surface, bumps rounded on dorsum, tapered on ventrum. Antenna about 1.5 times as long as wide. Posterolateral apodeme 2.2 times longer than head capsule. Spatula with slender shaft and two triangular anterior lobes separated by wide notch. On each side of spatula asetose sternal papilla, two inner lateral papillae with strong setae, and one asetose outer lateral papilla. Pleural and dorsal papillae with long setae. Terminal segment with six papillae, two situated ventrally next to anus, all with strong setae.
Pupa. – Unknown.
Other material examined – All material from Israel, Akko , collected by N. Dorchin from stem galls of Atriplex portulacoides unless otherwise noted. 2 Ψ, 3.III.1995; 4 larvae, 9.III.1999; 2 Ψ, 2 ɗ, 7.IX.2002, A. Freidberg; 3Ψ, 1ɗ, 10.IX.2008, A. Freidberg.
Distribution. – Israel (Na’aman salt marsh), Italy, UK, Portugal, Czech Republic.
Etymology: The name brevipalpis is Latin for short palpus. Kieffer (1898) indicated that the second segment of the palpus is about half as wide as the first, an attribute that allegedly sets the species apart from its congener, S. atriplicis .
Biology: The species induces single or multi-chambered galls in stems, leaf petioles or midribs of Atriplex portulacoides ( Figs. 9–10 View FIGURES 5 – 10 ). Galls are not common and are sometimes very difficult to find, although they can be locally abundant on several adjacent plants. Although the host is abundant in other localities in Israel (e.g., in the Atlit salt marsh and along the banks of the Yarkon river in Tel Aviv), galls were found only in the Akko salt marsh. The galls constitute 1–3cm wide swellings of variable shapes and are mostly green, but sometimes have a touch of red. First and second instar larvae are found in chambers lighter in color than the remainder of the gall tissue. Third instar larvae are found individually in rigid capsules inside the galls, where they spin a white cocoon before pupating. The chamber is sealed inside the gall by a round, thin, whitish disk that appears to be derived from silk. Pupae were not found and adults were extremely difficult to rear, both due to high parasitism rates and to a very short emergence season. However, the fact that adults were reared both in March and in August-September suggests that the species has at least two generations a year.
Remarks. – The genus Stefaniella includes 9 described species, all but one are restricted to saltbushes ( Atriplex spp.). Three species, S. atriplicis Kieffer , S. brevipalpis , and S. trinacriae Stefani , were described from salt-marsh habitats in the Mediterranean area ( Kieffer 1898, De Stefani 1900), whereas the remaining six were described from hosts that typically grow in arable or disturbed habitats in central Asia or in Italy and Eastern Europe ( Gagné 2004).
Stefaniella brevipalpis View in CoL is the only species recorded from A. portulacoides View in CoL . Although Houard (1908) lists A. halimus View in CoL as a host, we never found S. brevipalpis View in CoL galls on this plant, so this record may be erroneous. Stefaniella brevipalpis View in CoL galls are much smaller and more regular in shape than the amorphous galls of S. trinacriae View in CoL on A. halimus View in CoL , which often exceed 5 cm in length (N. Dorchin, unpublished data). The larvae of S. brevipalpis View in CoL are orange, whereas those of S. trinacriae View in CoL and S. altiplicis (both on A. halimus View in CoL ) are white to pale yellow ( Kieffer 1898, 1912, De Stefani 1900). Kieffer (1898) stated that the larval spatula differs between S. brevipalpis View in CoL and S. atriplicis View in CoL but did not elaborate. Another alleged difference between these species is the thinner second segment of the palpus in S. brevipalpis View in CoL , but we did not find the palpus in this species to be different from that of congeners we examined. We did find a good morphological difference between the ovipositors of S. brevipalpis View in CoL and S. trinacriae View in CoL , namely, a posterior extension of the lateral plate in the latter that is absent in the former ( Fig. 51 View FIGURES 45 – 53 ).
While S. trinacriae appears to stand out from the other two species both with regard to adult and gall morphology, S. brevipalpis and S. atriplicis are possibly synonymous. We examined adults reared by Fedotova from Atriplex longifolia , which allegedly belong to S. atriplicis , and could not find any morphological differences between them and the adults we reared from A. portulacoides . Examination of type material of two central-Asian species, S. usjurtensis Fedotova and S. gigantea Fedotova , did not reveal any morphological differences as well, other than the larger size of S. gigantea adults. Given the remarkable morphological uniformity of Stefaniella adults, a study of the immature stages is highly warranted, but it is likely that only a molecular analysis would allow determining the validity of species and the relationships among them. Until such analysis is conducted, the best characters for distinguishing among Stefaniella species are those of their galls.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Stefaniella brevipalpis Kieffer, 1898
| Dorchin, Netta & Freidberg, Amnon 2008 |
Stefaniella brevipalpis
| Kieffer 1898: 56 |