Laeliaena, AND
|
publication ID |
https://doi.org/10.1111/zoj.12402 |
|
persistent identifier |
https://treatment.plazi.org/id/06558E4F-FFF5-FFDF-C701-55E6FD483ACE |
|
treatment provided by |
Marcus |
|
scientific name |
Laeliaena |
| status |
|
LAELIAENA AND View in CoL LIMNEBIUS
Parameres
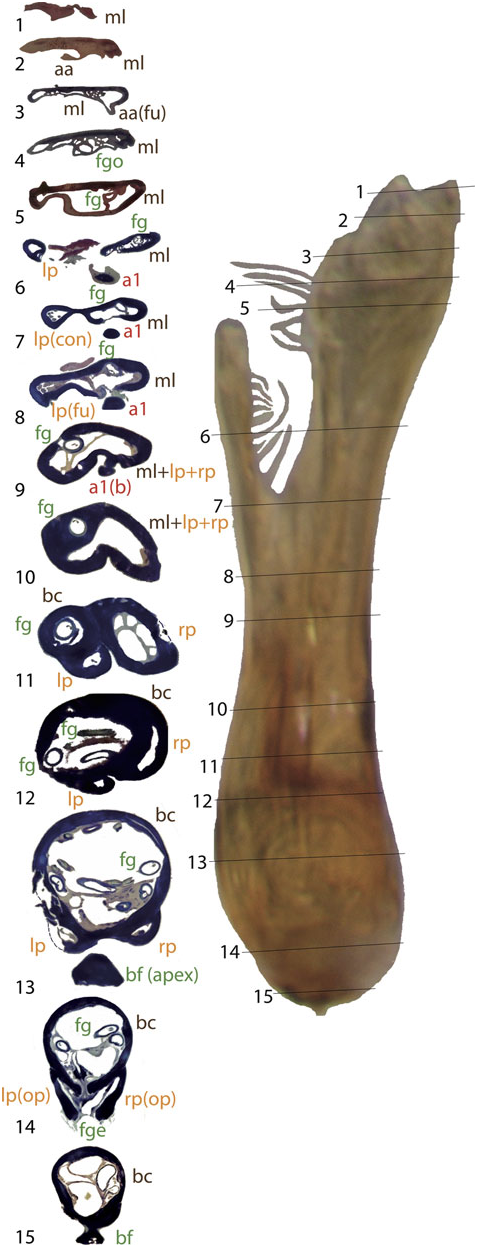
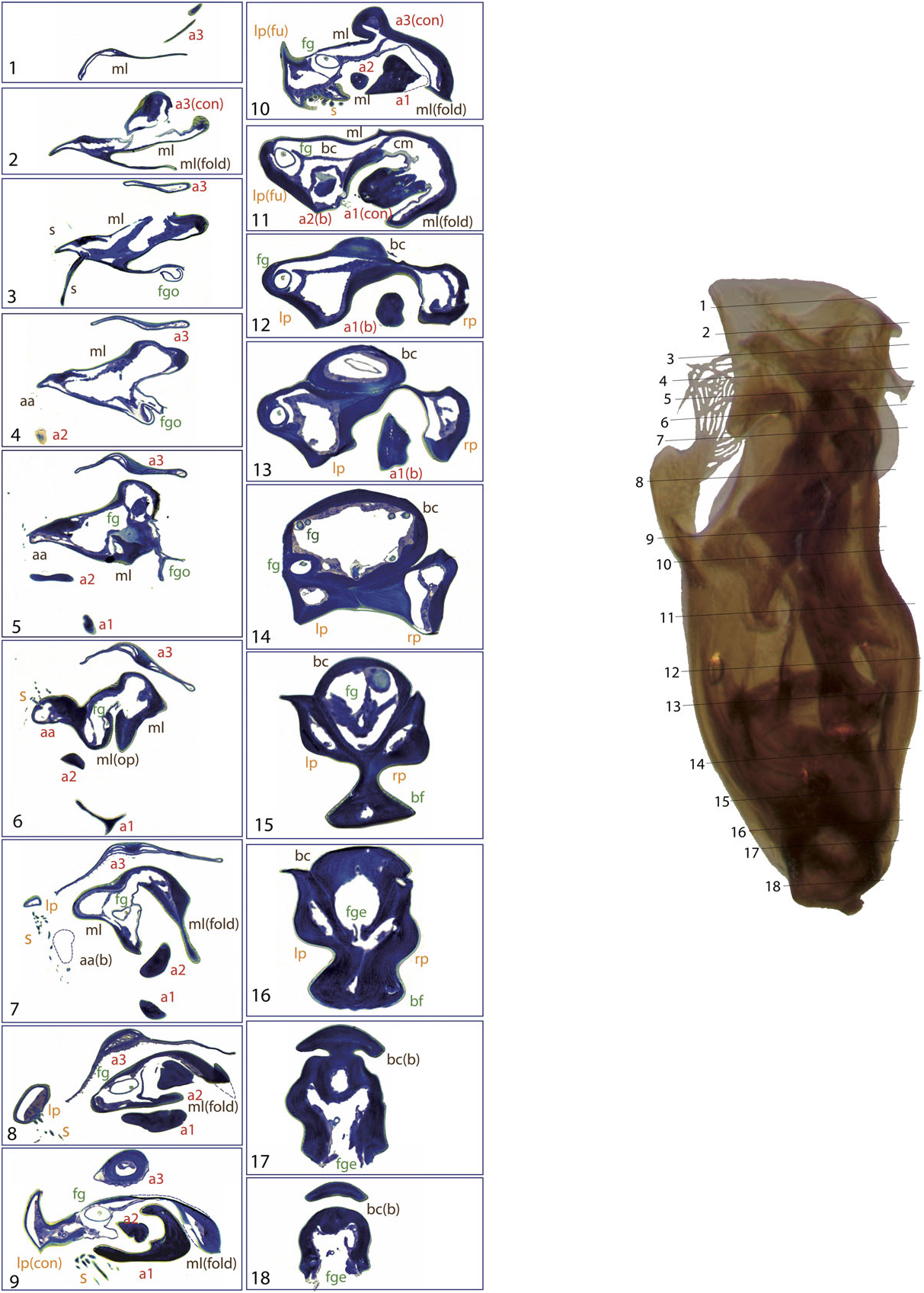
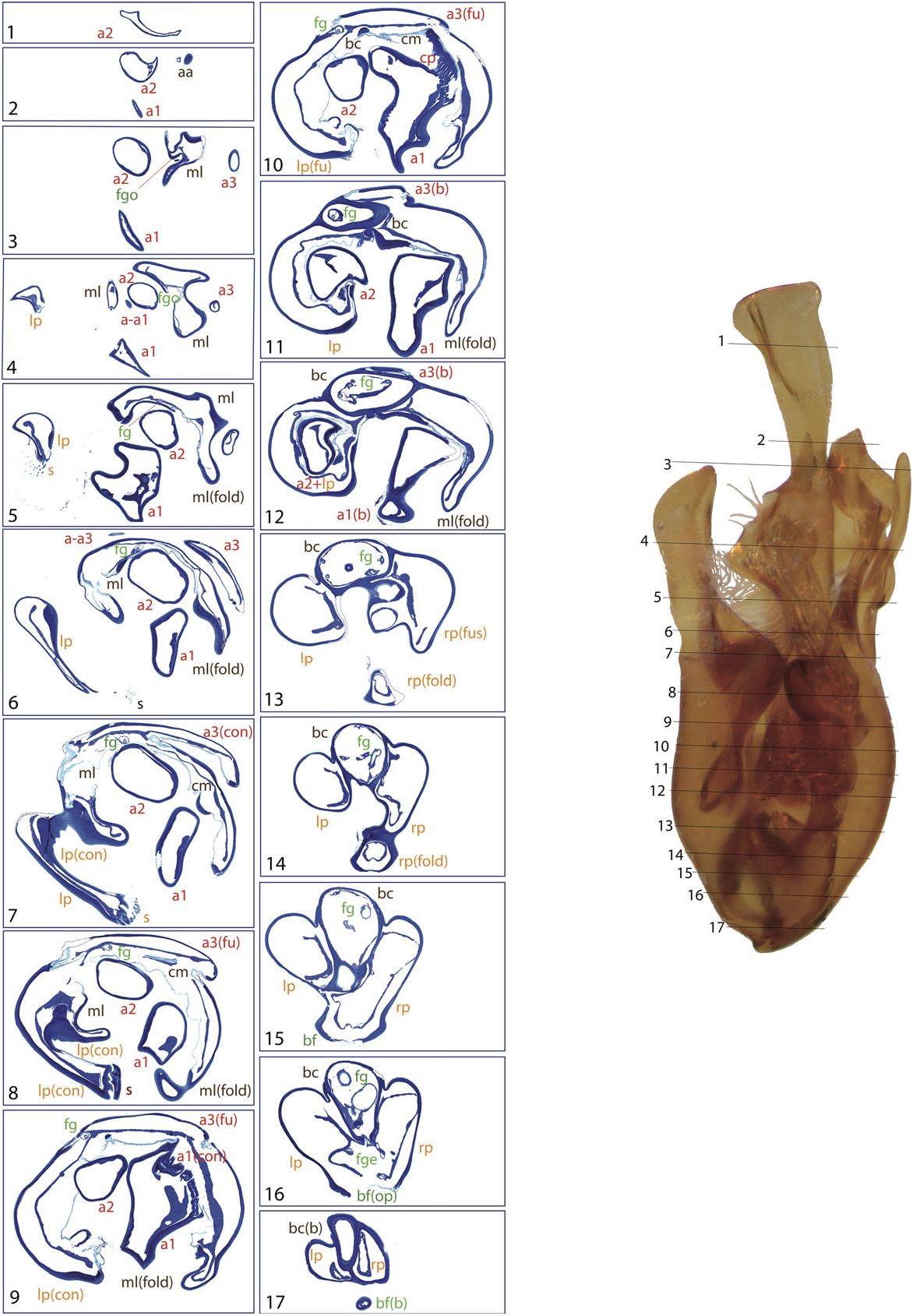
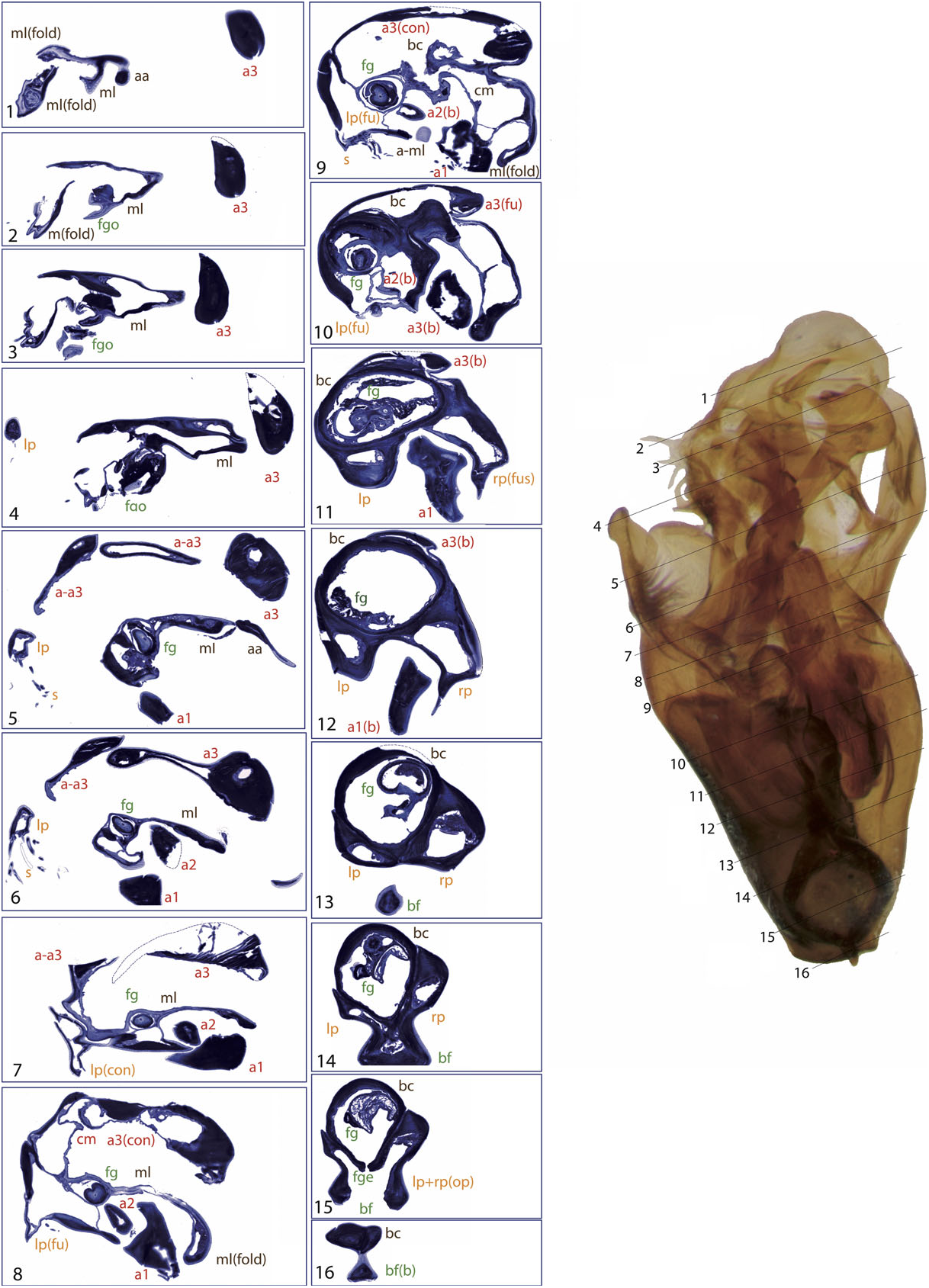
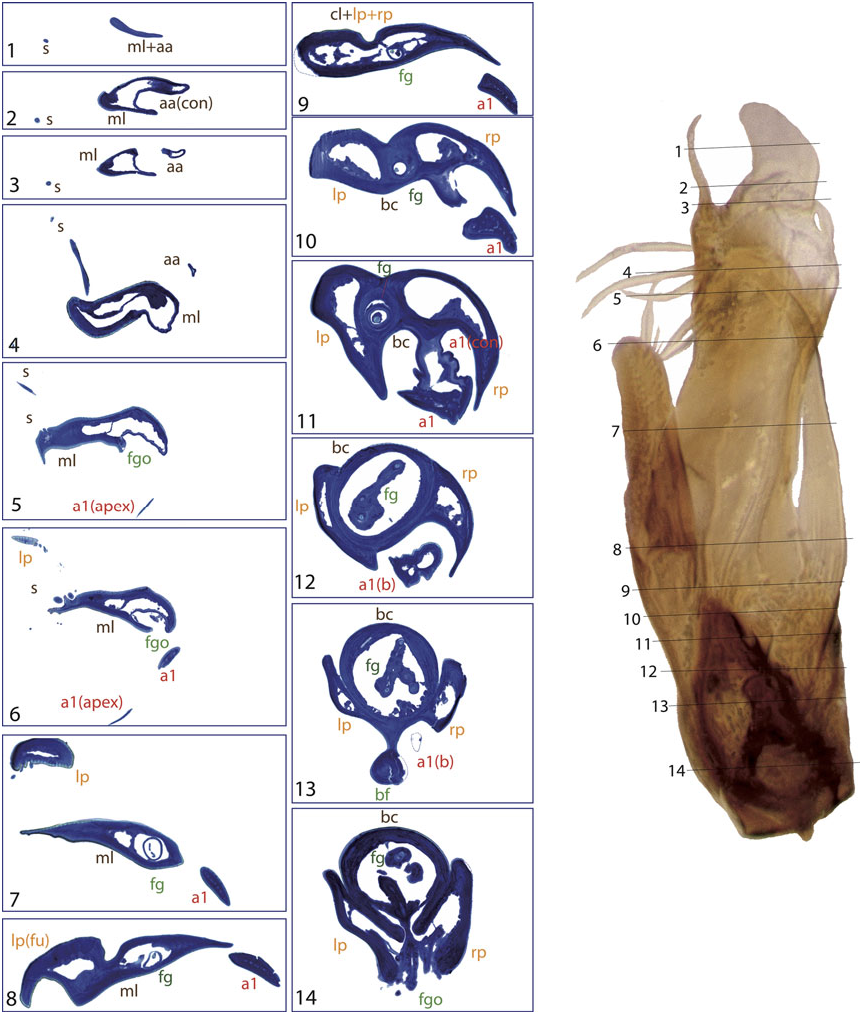
The general structure of the aedeagus in Coleoptera (see, e.g., Beutel & Lawrence, 2005) can still be recognized in Limnebiini , although it is usually highly modified. The basal piece (phallobase) is fused with the median lobe (penis), and the parameres (lateral lobes) are also partially or completely fused with them. In all species with serially sectioned specimens the bases of the two parameres were clearly recognizable as separate cavities, generally symmetrical, on both sides of a central structure corresponding to the median lobe ( Figs 4 – 10 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 View Figure 9 View Figure 10 ). In all studied species the lumen of the base of the parameres is connected to the foramen (see below; see also e.g. Figs 10 View Figure 10 :12 – 13, 6:17, 8:16).
In Laeliaena both parameres are fused with the median lobe, although they are still externally recognizable, and both have free apices always bearing setae (J ach €, 1995; Fig. S2 View Figure 2 :1 – 3). In most species of Limnebius the right paramere is completely fused with the median lobe and cannot be externally identified (we follow J ach €, 1993 in the orientation of the aedeagus). In species with serially sectioned specimens the lumen of the right paramere could only be clearly identified at the base of the aedeagus. In most studied species the lumen of the right paramere fuses completely with that of the median lobe shortly after the base (e.g. Figs 4 View Figure 4 :13, 5:10, 10:9). Externally, it can only be recognized as a small apical appendage in some species of Bilimneus (e.g. L. boukali J ach € or, to a lesser degree, L. rufipennis Regimbart , Fig. S2 View Figure 2 :11,51). In the species of the L. parvulus group the fusion of the cuticle of the right paramere with that of the median lobe is almost complete, but there is an appendage on the right side of the median lobe immediately above the fused area that could be interpreted as the apical part of the right paramere. Due to the lack of setae or visible pores on this appendage (as observed with SEM in L. stagnalis Guillebeau , Fig. 11 View Figure 11 ) we have not considered it as a visible right paramere, in agreement with J€ ach (1993). However, the homology of this structure remains uncertain (see ‘Additional appendages’ below; Figs 6 View Figure 6 :9,10, 11). Other species have also an appendage on the right side of the aedeagus (e.g. L. fretalis and L. nitiduloides , Figs 7 View Figure 7 , 8 View Figure 8 ; also present, but less apparent, in L. ferroi J ach € or L. mesatlanticus Thery , Fig. S2 View Figure 2 :77,127). However, due to the complete fusion of the right paramere with the cuticle of the median lobe below the origin of this appendage (in species with serial sections) and its different anatomical position, the interpretation of these appendages as a possible continuation of the right paramere is less convincing. In some species a group of setae is present on the right side of the apical part of the median lobe (J ach €, 1993), but the homology with a true right paramere remains also uncertain.
In clear contrast, the distal part of the left paramere is still recognizable in most species of Limnebius s.s. Its shape can vary from relatively long and curved, covering most of the dorsal part of the aedeagus (as in e.g. the species of the L. parvulus group; Fig. 11 View Figure 11 ) to short and straight (as in e.g. the species of the L. nitidus group) ( Figs 2 View Figure 2 , 4 – 10 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 View Figure 9 View Figure 10 ). Even when the left paramere is partially fused with the median lobe (e.g. in the L. piceus group, Perkins, 1980; Fig. S2 View Figure 2 :145,149,158) or even completely (as in species of the L. mundus group), there is still a recognizable group of setae inserted on the apical or subapical region of the entire structure, suggesting the inclusion of a remnant of a paramere (as shown with serial sections, see below). In the species of Bilimneus , also with a completely fused left paramere, there are also always some setae on the left side of the median lobe. They probably correspond to the apex of the paramere (J ach €, 1993).
The middle region of the left paramere can also be partly fused with the median lobe, with the two lumina only separated by a thin layer of cuticle in some species (e.g. L. fretalis , Fig. 7 View Figure 7 :8 – 11, or L. nitiduloides , Fig. 8 View Figure 8 :13). In the studied species of the L. punctatus and L. truncatellus subgroups the cuticle of the left paramere simply fuses with that of the median lobe ( Fig. 9 View Figure 9 :9), while in the L. nitiduloides subgroup the cuticle of the paramere is connected with that of the median lobe in a more complex form ( Fig. 8 View Figure 8 :12).
Ejaculatory duct (‘flagellum’)
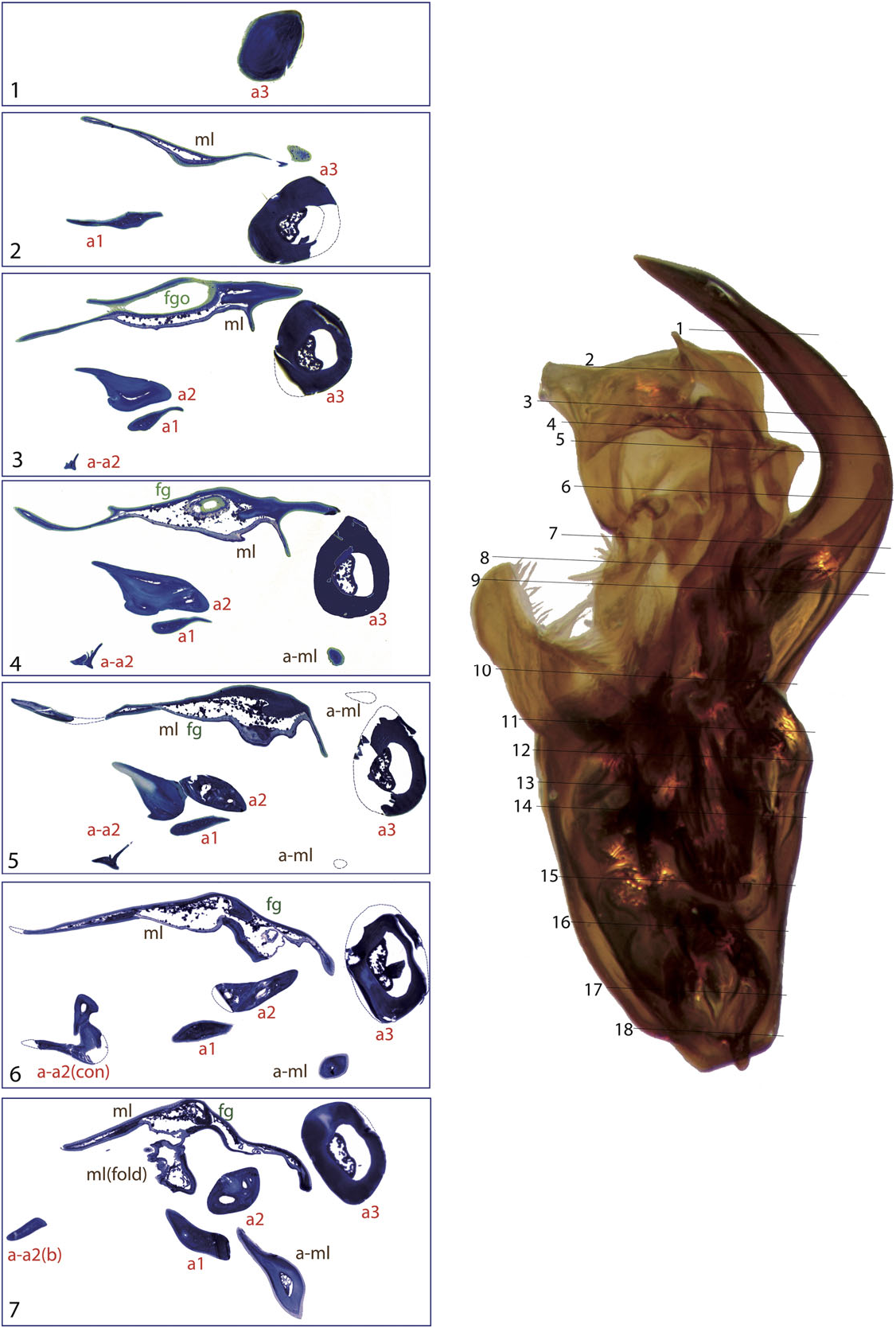
A strongly sclerotized ‘flagellum’ is present in all species of Limnebini. In Limnebius s.s. it is coiled within a cavity at the base of the median lobe (the ‘basal capsule’, see below) ( Perkins, 1980; J ach €, 1993, 1995). In species of the L. parvulus subgroup ( Fig. 4 View Figure 4 ; Fig. S2 View Figure 2 :132,141) this basal capsule is very distinct, with multiple loops of the flagellum in its interior. The flagellum can be partly fused with the upper part of the basal capsule (see e.g. Figs 5 View Figure 5 :10,11, 6:15,16, 10:10,11).
The flagellum exits the median lobe through an opening usually located in its apical third, in some cases forming a small channel ( Fig. 4 View Figure 4 :5). In some species the opening is surrounded by or near to a group of setae (e.g. L. parvulus group, Fig. 4 View Figure 4 :5), or it is located on a small prominence of the median lobe (in the L. truncatellus and L. punctatus subgroups, Figs 6 View Figure 6 :3 – 5, 9:3,4). In the L. nitiduloides subgroup the relative position of the flagellum opening is less apical, without any special prominence, but associated with different structures. Thus, in L. nitidu- loides it is dorsal and with a lamina ( Fig. 8 View Figure 8 :3), while in L. fretalis it is small and placed inside a special fold of the median lobe ( Fig. 7 View Figure 7 :4). In the species of Bilimneus the flagellum opening is always nearly apical, without any well-defined structure of the median lobe beyond it.
The flagellum is normally retracted inside the aedeagus and only rarely seen extended. Perkins (1980) noted that among more than 1000 studied specimens only one had it extended (in L. ozapalachicus Perkins , fig. 75F in Perkins, 1980). Even though the holotype of L. endroedyi Perkins is depicted with an everted flagellum in Perkins (2015), it is noted that the usual condition of the species is with this structure retracted. J ach € (1993) also noted that among all the material he studied only one specimen had an extended flagellum, in the species L. kweichowensis Pu (see fig. 19b in J ach €, 1993). In Ferro (1989) L. cuspidatus Ferro (currently a synonymy of L. atomus , J ach €, 1993) is illustrated with an everted flagellum, which was interpreted as a filiform paramere by the author. Among all the material examined we found only one specimen of L. hieronymi Vorst with extended flagellum ( Fig. S2 View Figure 2 :104). Among the species newly described from South Africa by Perkins (2015) one, L. masculinus Perkins , seems to have a permanently everted apex of the flagellum. However, as noted by the author, the homology of this exposed structure with the internal flagellum is not well established.
In all species with histological sections the flagellum can be distinguished as a tubular structure, hollow and well sclerotized, with sometimes some undifferentiated cells or tissue in its interior (e.g. Figs 6 View Figure 6 :17, 9:11). The function of the flagellum is thus likely to transfer sperm during the copula, i.e. it can be considered an ejaculatory duct, similar to that found in other beetle groups (e.g. Rodrıguez, Windsor & Eberhard, 2004). It does not seem to be a mechanical aid for the copula, as can be the case in other groups of Coleoptera with similar structures (e.g. Scydmaeninae, Jałoszynski et al., 2015). Presently there are no data on the mechanics of the copula in Limnebius , and the mechanism by which the flagellum is everted is unknown. Perkins (1980) noted that Limnebius females have a long tube between the bursa copulatrix and the spermatheca, which agrees with our observations (see above). This could suggest that at least a part of the flagellum is inserted in the female genital track during the copula.
Median lobe
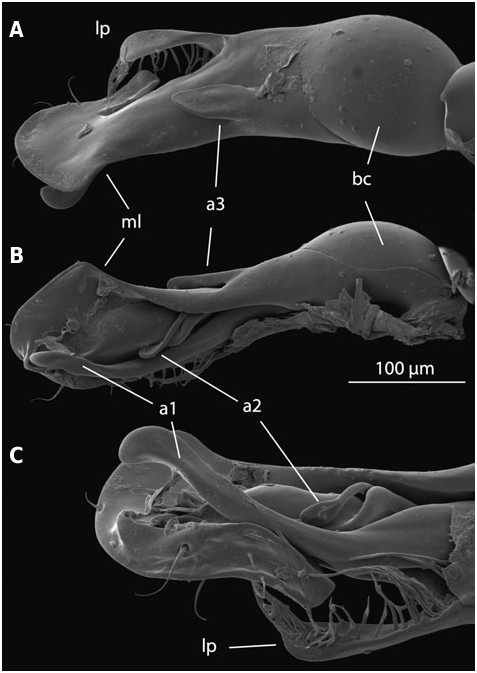
The median lobe is asymmetrical in all species of Laeliaena and Limnebius . In most species of Bilimneus it is either straight or tends to be curved to the left, while in most species of Limnebius s.s. it tends to be curved to the right (as in e.g. the L. nitidus subgroup). However, there are exceptions in both lineages ( Fig. 2 View Figure 2 ; Fig. S2 View Figure 2 :77,78,106).
In all species of Limnebius a spherical or oval hollow capsule is present inside the median lobe. This capsule is basal and the flagellum coiled inside in species of Limnebius s.s. (the ‘basal capsule’). In contrast, it is placed medially or apically in the species of Bilimneus , with a different shape and with an uncoiled flagellum ( Fig. 2 View Figure 2 ). In L. feuerborni (included in the clade B1 of Bilimneus , Fig. 3) the main cavity of the median lobe is larger and in a more basal position within the median lobe than in all other species of Bilimneus (d’Orchymont, 1932; Fig. S2 View Figure 2 :27). However, it has a different structure than in species of Limnebius s.s. and does not reach the base of the aedeagus.
The basal capsule is usually strongly sclerotized in the species of Limnebius s.s. (e.g. Figs 2 View Figure 2 , 9 View Figure 9 ). In some species the upper part of the basal capsule can be flattened, divided by thin cuticular walls (as in e.g. some of the species with the most complex aedeagus of the L. nitidus group, Figs 7 View Figure 7 :10, 9:9) or even subdivided completely (e.g. in L. nitiduloides , Fig. 8 View Figure 8 :12).
In the distal part of the median lobe, above the capsule, the lumina of the parameres are usually totally (as in e.g. L. furcatus , Fig. 4 View Figure 4 :12,13) or at least partially fused with the lumen of the median lobe. The lumen of the right paramere is separated from that of the median lobe by thin cuticular walls in some species, with a poorly defined structure (e.g. L. pilicauda , Fig. 6 View Figure 6 :10). Usually its distal part is completely fused with the cuticle of the median lobe (e.g. L. fretalis , Fig. 7 View Figure 7 :9,10, or other species of the L. nitidus group, Figs 10 View Figure 10 :9, 8:12). It is thus uncertain whether the appendages or other structures originating from the walls of this cavity are homologous with the right paramere (see above). Regardless, we use the presence of the flagellum to identify the central cavity resulting from the complete fusion of the med- ian lobe and the right paramere. There are some differences in the anatomical position of this fused right paramere + median lobe among the lineages of Limnebius s.s. In the L. parvulus group (represented by L. furcatus ) it lies on the right side of the aedeagus, which is strongly flattened dorsoventrally and constricted in the central region ( Fig. 4 View Figure 4 :13,14). In other groups it is placed in a more central position (e.g. L. nitidus group, Fig. S2 View Figure 2 :81,96).
The apical part of the median lobe, beyond the opening of the flagellum, extends and forms different structures in most species of Limnebius s.s. In some species (as in e.g. L. papposus or L. doderoi Gridelli within the L. parvulus group, Fig. S2 View Figure 2 :33,90) they are large and cover most of the apex of the aedeagus, but in others (mostly within the L. nitidus group) they are thin and more lateral (e.g. L. truncatellus , Figs 2 View Figure 2 , 9 View Figure 9 ). In species of Bilimneus the apex of the aedeagus is usually simple, although in some the apical part can have some hook-like small appendages (e.g. L. pollex J€ ach & Delgado; J ach € & Delgado, 2013; Fig. S2 View Figure 2 :47).
Basal foramen
In all species of Limnebiini , as in other genera of Hydraenidae , the ejaculatory duct enters the aedeagus through a ventral opening at the base, the basal foramen (the ‘median foramen’ of Sharp & Muir, 1912). The structure of the basal foramen is very conserved, with a strongly sclerotized ring with a peg-like structure in the distal part and a basal pointed projection (J€ ach, 1993; Fig. 2 View Figure 2 ). The conservation of this structure also affects the base of the parameres, the lumina of which open into the foramen in all studied species (see above). The only variation refers to its general shape: in Laeliaena and most species of Bilimneus the foramen is oval or somewhat triangular (with some exceptions, e.g. L. pararabicus J ach € & Delgado, L. rufipennis or L. taiwanensis J€ ach), whereas it is round in most species of Limnebius s.s. (with the exception of some species of the L. mundus group, see below) ( Table S1, Fig. S2 View Figure 2 ).
The relative position of the foramen varies with the shape of the base of the aedeagus; in species with a well-developed capsule (e.g. within the L. parvulus group, Figs 4 View Figure 4 , 11 View Figure 11 ) it is more lateral and in a more distal position, while in species with a straight base (e.g. some species of Bilimneus , Fig. 2A View Figure 2 ) it is almost at the base of the aedeagus.
Additional appendages
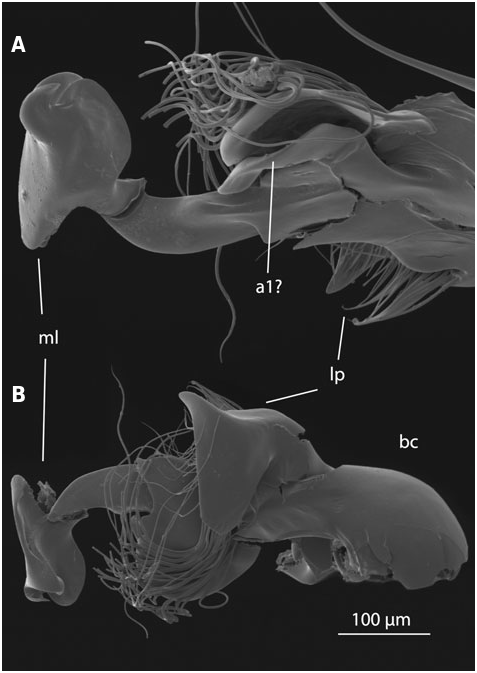
Additional appendages of the aedeagus are present in many species of Limnebius s.s., resulting in the highly complex male genitalia typical for the genus (J ach €, 1993). These appendages can be distinguished based on their position on the ventral or dorsal side and on their origin. In the species with the most complex aedeagus they may have secondary subdivisions. One or two main ventral appendages can occur (a1 and a2, corresponding in most cases to appendages A and B in J ach €, 1993), and one dorsal (a3, corresponding to appendage C in J ach €, 1993). All of them can be subdivided. In most cases we have identified these appendages based on their position but also on structural features. However, the homology between appendages of different groups of Limnebius remain uncertain. In some species they may represent vestiges of the right paramere.
Appendage a1 is present in all species included in the L. gracilipes group and in most species of the L. nitidus group (except for the species of the L. mundus subgroup and some within the L. nitidus subgroup, Table S1). In almost all studied species appendage a1 has multiple internal channels (see e.g. the base of a 1 in L. nitiduloides , Fig. 8 View Figure 8 :8 – 14), with the only exception of L. fretalis , in which the channels fuse in a single lumen just after the base ( Fig. 7 View Figure 7 :2 – 14). Appendage a2 usually has a single internal cavity, although in L. nitiduloides the apical part shows a complex shape with multiple folds and cavities ( Fig. 8 View Figure 8 :3 – 7).
In the species of the L. nitidus subgroup appendage a1 forms the ‘pseudoparamere’ described by J€ ach (1993). Its elongate shape and general appearance led some authors to consider it as the right paramere, but histological sections show that this is not the case. This is in agreement with J ach € (1993), who based his interpretation mostly on the lack of setae or micropores, which are always present in true parameres. With our data it is not possible to test if this appendage was formed by fusion of setae, as suggested by J ach € (1993). However, the fact that in some species the appendage may be hollow for most of its length suggests that it may have originated as an extension of the cuticle, as it probably happened with other additional appendages. The similarities in the internal structure and the connection with the median lobe of the ‘pseudoparamere’ of the L. nitidus subgroup with the a1 of other studied species of the L. nitidus group (in particular L. truncatellus and L. pilicauda , Figs 6 View Figure 6 , 9 View Figure 9 ) support this interpretation. The ‘pseudoparamere’ may be functionally analogous to the right paramere in other species of Coleoptera , as it is placed in the same position and has a similar elongated structure. However, the function of the parameres (and other appendages) in Limnebius remains unclear.
The lumen of appendage a1 is always connected at some point to the central cavity of the median lobe, either by fusion of the cuticles (as in e.g. L. cordobanus , Fig. 4 View Figure 4 :9) or through an area with abundant small channels, as for instance in L. fretalis ( Fig. 7 View Figure 7 :10) or L. nitiduloides ( Fig. 8 View Figure 8 :11). In the species with small channels, apical to the area connected to the central cavity of the median lobe the cuticle of a1 tightly merges with that of the median lobe ( Fig. 8 View Figure 8 :12). In the species of the L. parvulus subgroup an appendage originates from the area where the right paramere fuses with the median lobe (see ‘Parameres’ above). In Jach € (1993) this is interpreted as a homologue of the ventral appendage a1 (his appendage A) in other species. Although its position and the internal structure are similar to that of a 1 in the species of the L. nitidus group, the histological sections of L. furcatus show that this appendage probably formed as an extension of the cuticle of the median lobe ( Figs 4 View Figure 4 :8 – 11, 11). An additional fold of the dorsal part of the median lobe is present in some species of the L. parvulus subgroup. This is apparently not an appendage, as its cuticle remains fused to the median lobe over its entire length ( Fig. 4 View Figure 4 :7 – 12).
The secondary ventral appendage (a2) is usually shorter and less curved than a1, without any specific modifications. In most cases it is located more laterally, with the base very close to that of the left paramere, or even surrounded by it in some species (e.g. Fig. 8 View Figure 8 :12 – 15). Appendage a2 only occurs if a1 is also present, in the L. nitiduloides , L. punctatus and L. truncatellus subgroups and in some species of the L. gracilipes group ( Table S1).
The lower part of both ventral appendages is usually enclosed in a deep groove of the median lobe, formed by the base of the parameres and the median lobe itself. This groove is most distinct in the L. nitiduloides subgroup, where ventral appendages are very strongly developed ( Figs 7 View Figure 7 :6 – 12, 8:8 – 13). In other species, such as those of the L. punctatus and L. truncatellus subgroups, the base of the parameres does not encircle a2, but only forms a widely open concavity ( Figs 6 View Figure 6 :10, 9:8 – 11).
In some species a1 and a2 intersect, as in L. pilicauda and others within the L. punctatus subgroup ( Fig. 6 View Figure 6 ). In these cases, a1 occupies a more central position on the aedeagus. Both a1 and a2 can form different structural types in the apical region, from short flap-like expansions of the cuticle (e.g. L. cordobanus , Fig. 4 View Figure 4 ) to long pectinated extensions (e.g. L. truncatellus , Fig. 9 View Figure 9 ).
The dorsal appendage a3 is present in all species of the L. truncatellus , L. punctatus and L. nitiduloides subgroups, and in some species of the L. gracilipes group ( Table S1). Its structure is similar to that of a1, although its base is different from both a1 and a2: it originates as a flat, thin extension of the dorsal side of the median lobe, actually forming its dorsal wall in the basal and middle regions ( Figs 7 View Figure 7 :8 – 12, 9:9 – 12). In most studied species the lumen of a3 is clearly visible. In L. pilicauda (and probably in other species of the L. punctatus subgroup, clade L4.2) the lumen is very narrow due to the dorsoventral flattening ( Figs 6 View Figure 6 :3 – 7, 12). In L. truncatellus it is reduced to a narrow channel due to the strong sclerotization and subdivision of the appendage ( Fig. 9 View Figure 9 :5,6). In L. pilicauda a3 is cylindrical at its base. It originates simply as an extension of the median lobe and flattens only in the apical region ( Fig. 6 View Figure 6 ), in contrast to the usual shape in all other species (base flattened and apex cylindrical). In L. mucronatus Baudi di Selve , also within the L. punctatus subgroup ( Fig. 3), a3 is only visible as a short flap-like structure on the dorsal side of the aedeagus, superficially resembling a short paramere ( Fig. 12 View Figure 12 ). In the L. truncatellus subgroup the distal region of a3 is divided to form three separate subappendages ( Fig. 9 View Figure 9 :5), two with a similar, flattened shape ( Fig. 9 View Figure 9 :5,6) and a cylindrical one that extends to the apex of the aedeagus ( Fig. 9 View Figure 9 :1).
The different types of connection of a3 with the median lobe suggest that it was formed independently in several lineages. It is central in the L. nitiduloides subgroup ( Fig. 8 View Figure 8 :14,15), inserted on the left side in the L. truncatellus subgroup ( Fig. 9 View Figure 9 :11,12), which is also characterized by a longitudinally subdivided apex, and of a less complex structure in the L. punctatus subgroup, where it lacks additional structures ( Figs 6 View Figure 6 :10, 11). Its presence seems to be strongly correlated with that of a2: of the 23 species of Limnebius with a2, only two lack a3 (both within the L. gracilipes group, Table S1).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.