Amaranthus retroflexus, L.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2019.01.016 |
|
DOI |
https://doi.org/10.5281/zenodo.10519549 |
|
persistent identifier |
https://treatment.plazi.org/id/0716880A-FFA4-3435-5C34-FC30C61B356C |
|
treatment provided by |
Felipe |
|
scientific name |
Amaranthus retroflexus |
| status |
|
2.2. Cytotoxicity of methanolic extracts of A. retroflexus View in CoL
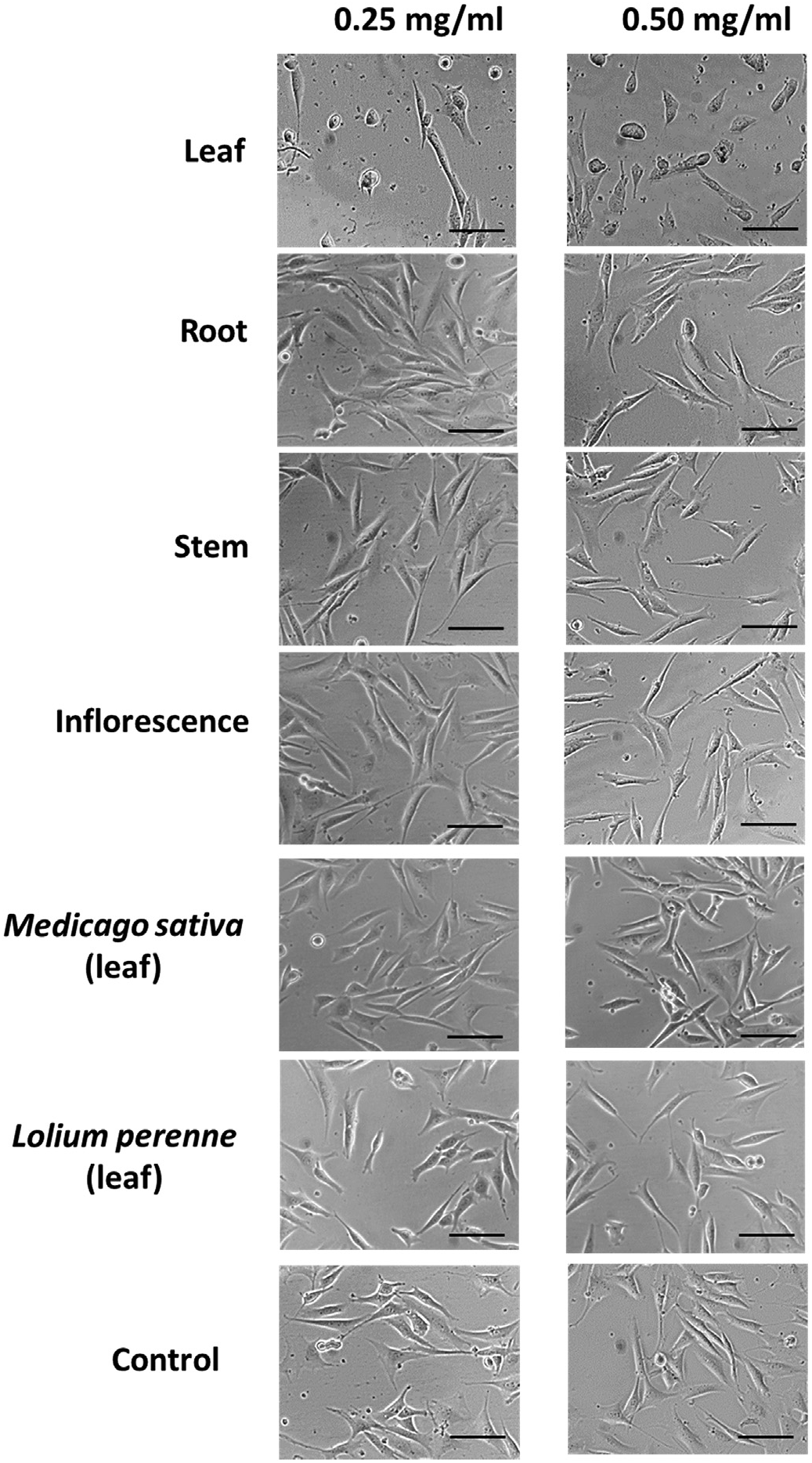
Exposure to A. retroflexus leaf extracts induced rapid cell death in NIH3T3 cells within two hours following treatment, with cells exhibiting typical apoptotic features including nuclear condensation and cell shrinkage ( Fig. 5 View Fig ). Control cultures treated with equivalent volumes of methanol (MeOH) showed very limited evidence of apoptosis, displaying characteristic fibroblast morphology and active cell division.
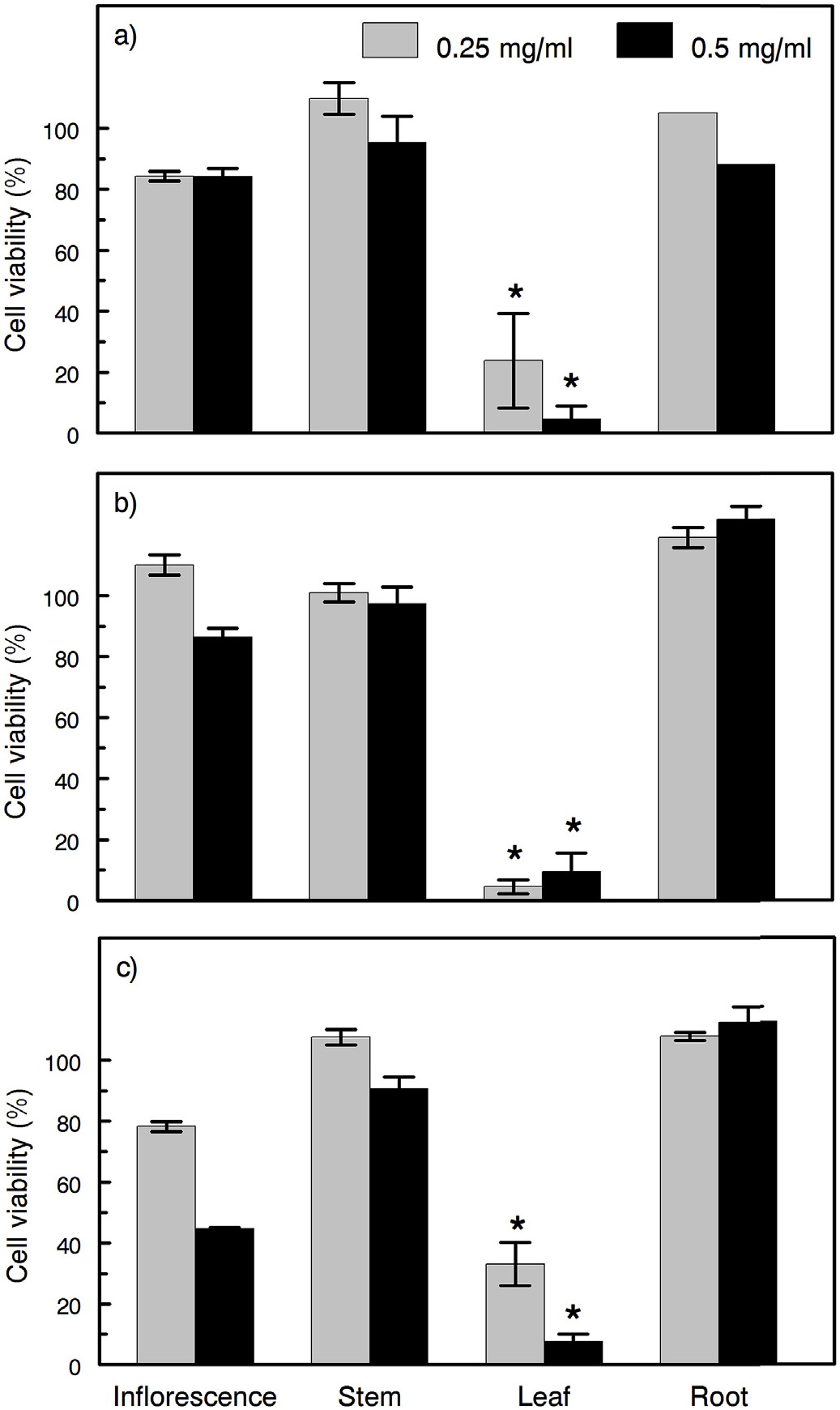
Exposure of NIH3T3 cells to extracts of A. retroflexus leaves from all three plant collections resulted in significantly higher cytotoxicity than extracts of other tissues obtained from the same plants. Cytotoxicity of leaf tissue extracts was observed at both concentrations examined (0.25 and 0.50 mg /ml) ( Fig. 6 View Fig ).
2.3. Identification of the toxic constituents of Amarathus retroflexus View in CoL extracts
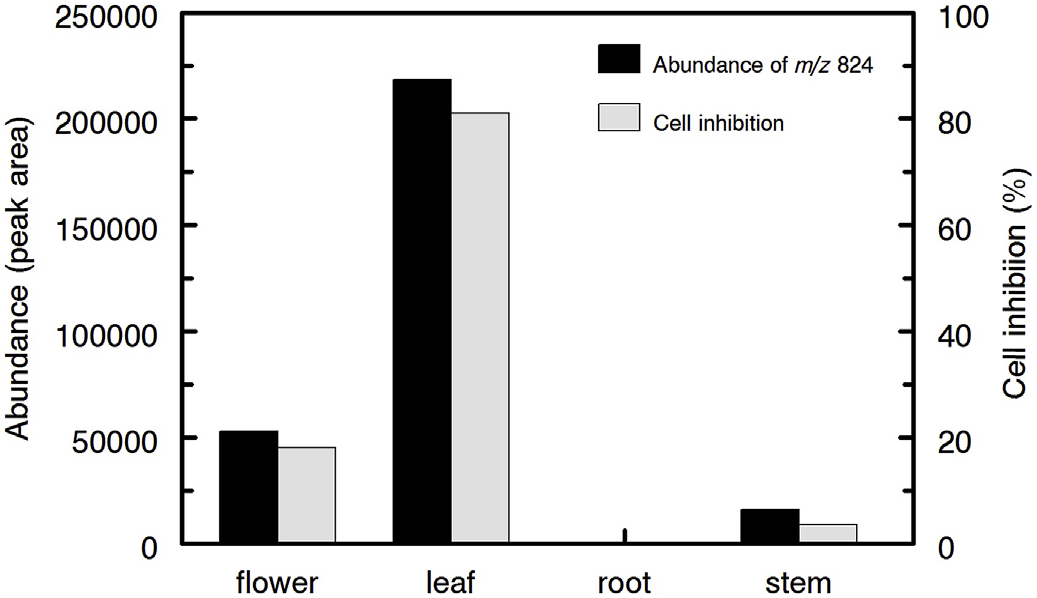
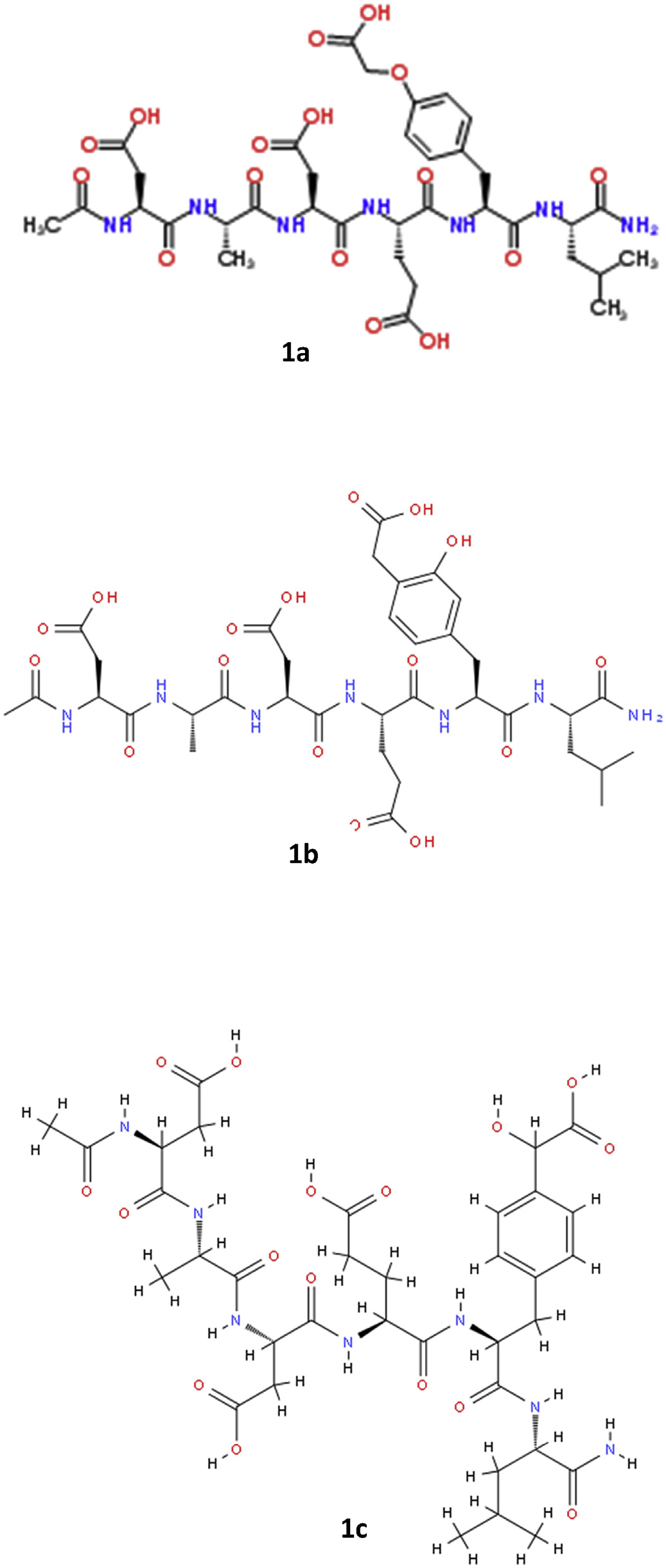
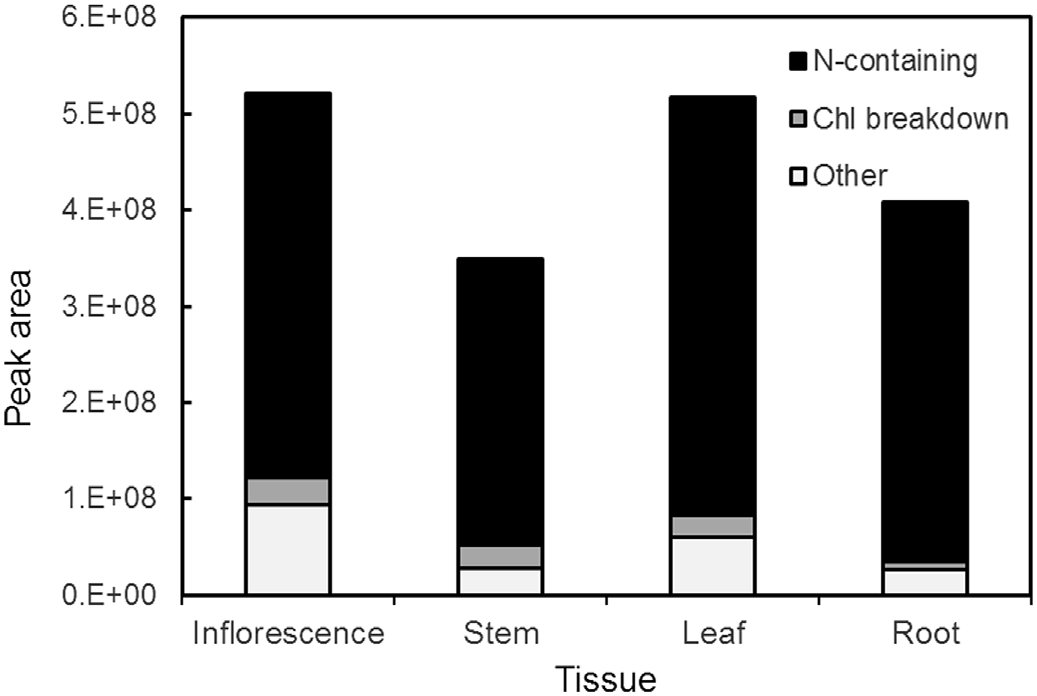
Despite the predominance of numerous nitrogen-containing compounds and chlorophyll derivatives in methanolic extracts of A. retroflexus , these compounds were not significantly or positively correlated with cytotoxicity. Stepwise linear regression using the relative abundance of compounds against in vitro cytotoxicity revealed no appreciable correlation between any components separated via HILIC chromatography and bioactivity. However, the cytotoxic activity in A. retroflexus leaf extracts was highly correlated at both concentrations examined [ 0.25 mg /mL ( r 2 = 0.928, P <0.001) and 0.5 mg /mL ( r 2 = 0.840, P <0.001)] with the presence of a single compound eluting on C 18 with m/z of 824.3300 ( Fig. 7 View Fig ). Given the very high coefficient of determination ( r 2) and the statistical significance of this match, we attempted to determine the structure of this potential toxin or toxicity biomarker by examining MS-MS spectra. The predicted molecular formula of this constituent was C 35 H 49 N 7 O 16. The use of Agilent Molecular Structure Correlator (MSC) applied to MS-MS spectra of this compound yielded a predicted 83% match with a small modified peptide, N-acetyl-L-α- aspartyl-L-alanyl-L-α- aspartyl-L-α- glutamyl-O- (carboxymethyl)-L-tyrosyl-L-leucinamide (structure 1a, Fig. 8 View Fig ). Two related modified peptides (1b and 1c, Fig. 8 View Fig ) also produced high scores via MSC, but their scores were somewhat lower (81 and 79%, respectively). The various software packages and libraries used for assistance with structural elucidation indicated that all candidate molecules as potential matches for the putatively active compound contained nitrogen and were small modified peptides. The MS-MS spectra of the putatively active compound under collision energies of 10, 20 and 40 eV is shown in Fig. S1. View Fig View Fig View Fig
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |