Aletheiana, Ng, Peter K. L. & Lukhaup, Christian, 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.4039.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:A1F46EFE-0639-4754-9F45-69915F2599D9 |
|
DOI |
https://doi.org/10.5281/zenodo.6106629 |
|
persistent identifier |
https://treatment.plazi.org/id/08492243-FFA1-FF8F-6D87-EB86327098D5 |
|
treatment provided by |
Plazi |
|
scientific name |
Aletheiana |
| status |
gen. nov. |
Genus Aletheiana View in CoL gen. nov.
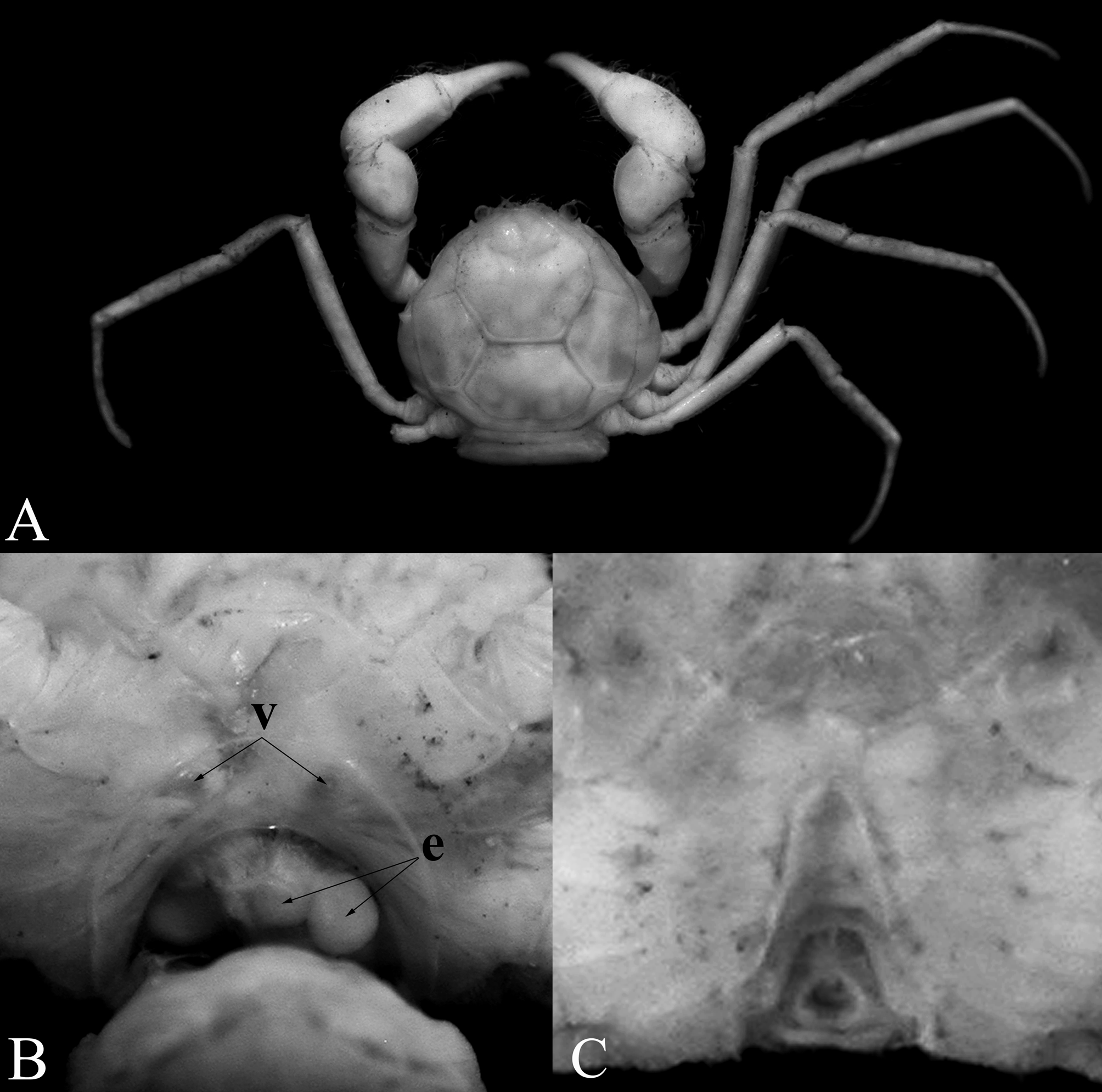
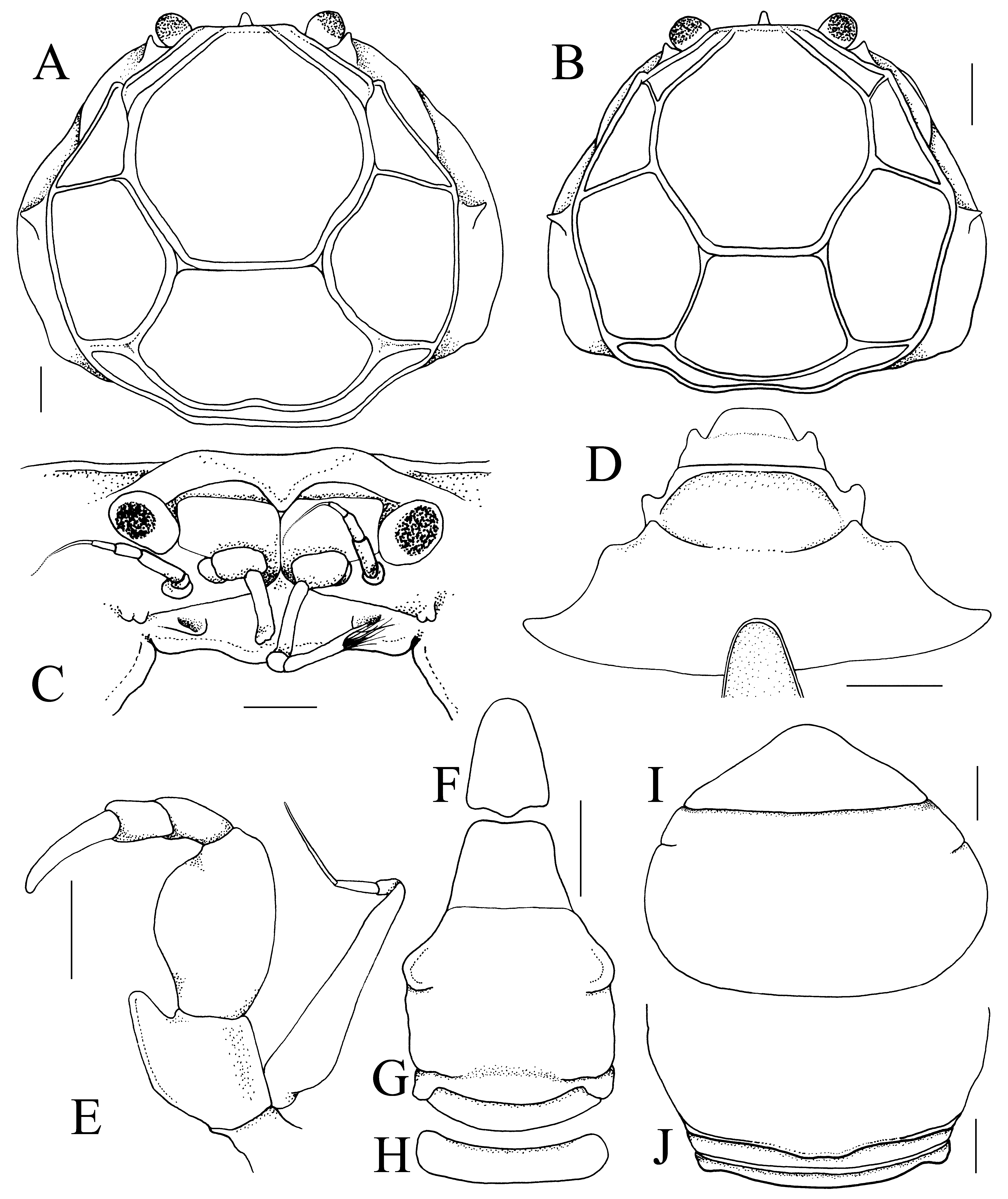
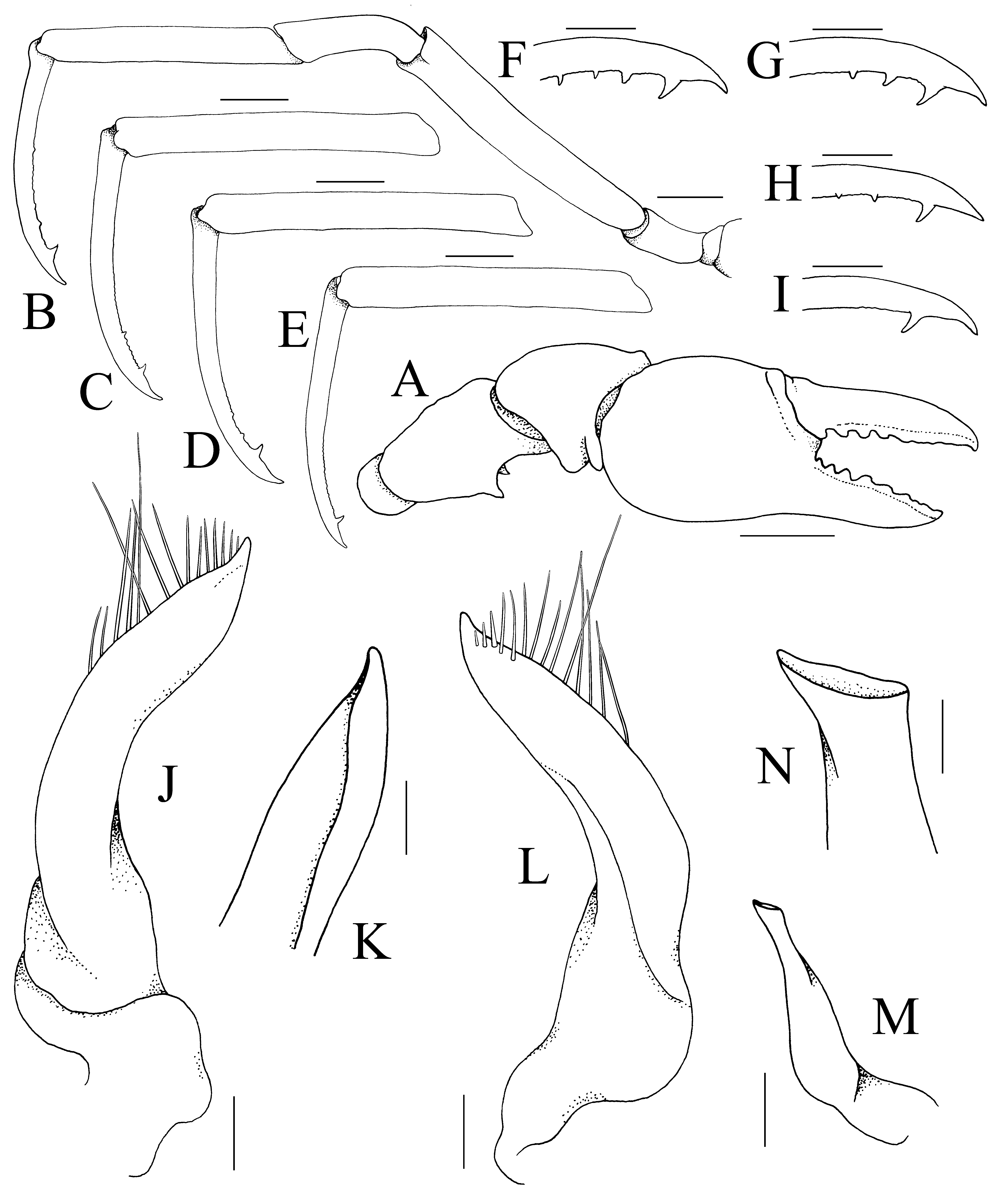
Diagnosis. Carapace subovate, wider than long, dorsal surface surrounded by continuous rim, regions clearly separated by distinct grooves ( Figs. 2 View FIGURE 2 A, 3A, B); front entire, only small median lobe submarginal in position, visible from dorsal view ( Fig. 3 View FIGURE 3 A–C); no discernible orbit; eye pigmented, mobile, visible from dorsal view (Fig. A–C); antennular fossa shallow, basal article short, quadrate with second and third articles elongate ( Fig. 3 View FIGURE 3 C); antenna with long, filiform flagellum, base positioned between base of ocular peduncle and edge of antennular fossa ( Fig. 3 View FIGURE 3 C); epistome nearly vertical in lateral view, relatively broad, posterior margin with 2 low median rounded lobes ( Fig. 3 View FIGURE 3 C); third maxillipeds narrow, forming wide gape when closed, merus elongated, ischium short, long distal inner extension, dactylus elongated, twice length of propodus ( Fig. 3 View FIGURE 3 E); ambulatory dactylus armed with prominent subdistal spine ( Fig. 4 View FIGURE 4 B–I); sterno-abdominal cavity without locking button; male abdomenpleotelson slender, elongated, 4-segmented, somites 3 and 4 fused, somite 5 immobile, separated from somite 4 by shallow suture, telson linguiform, elongated ( Fig. 3 View FIGURE 3 F–H); G1 medially twisted, tip sharply tapering, turned upwards, dorsal side of inner margin lined with long, stiff setae ( Fig. 4 View FIGURE 4 J–L); G2 with base not dilated, tip subspatuliform ( Fig. 4 View FIGURE 4 N, M); female with wide cephalothorax cavity, margin of cavity connected to that of abdomen-pleotelson by membrane, forming internal brood cavity ( Fig. 2 View FIGURE 2 B); female abdomen with somites 3–5 completely fused, pleotelson free ( Fig. 3 View FIGURE 3 I, J).
Etymology. “ Aletheia ” is an ancient-Greek word for disclosure and a state of not being hidden. The latinised name is used here as a genus, alluding to the present discovery of the first free-living hymenosomatid crab from Sulawesi. Gender of genus feminine.
Remarks. Aletheiana gen. nov. shares characters of Neorhynchoplax Sakai, 1938 (type zzz species Rhyncoplax introversus Kemp, 1917 ) and Sulaplax Naruse, Ng & Guinot, 2008 (type species Sulaplax ensifer Naruse, Ng & Guinot, 2008 ). Members of Neorhynchoplax are defined by the following suite of characters: the presence of a well developed rostrum, well developed eyes which are mobile, the base of the antenna being positioned below the base of the ocular peduncle and antennular fossa, the median part of the posterior margin of the epistome is formed by a distinctly triangular plate, relatively narrow third maxillipeds which do not not cover more than three-quarters of the buccal field when closed, the merus of the third maxilliped is usually ovate and not elongated, with the ischium relatively long, and the dactylus short and as long as or slightly longer than the propodus, the male abdomen is usually broadly triangular, and a simple G1 which has the distal part tapering and not armed with lobes and processes (cf. Ng & Chuang 1996; Naruse & Ng 2007; Naruse et al. 2008b; present data). The monotypic Sulaplax also has narrow third maxillipeds, and the structures of the carapace, male abdomen and G1 are similar, except that it has only a vestigial rostrum (the vestigial median lobe is very small and not visible from dorsal view), the eyes are immobile and degenerated, the base of the antenna being positioned more laterally beyond the antennular fossa, the median part of the posterior margin of the epistome consists of two broad lobes separated by a shallow depression, the merus of the third maxilliped is elongated with the ischium short and the dactylus elongated and much longer than the propodus ( Naruse et al. 2008b: 26–27; present data).
The loss of pigmentation in the eye, reduction in size of the cornea and fusion with the cephalothorax, as well as elongated chelipeds and ambulatory legs are probably features associated with a troglobitic lifestyle, and so may not be useful phylogenetic characters (see Holthuis 1986; Guinot 1988; Klaus et al. 2013; Ng 2013). The other characters, however, are less likely to be associated with cave adaptations.
The taxonomy of Neorhynchoplax Sakai, 1938 , has been discussed at length by Ng & Chuang (1996) and currently contains 32 species, all from the Indo-West Pacific (Ng et al. 2008; Naruse et al. 2008a; Ng et al. 2011). Aletheiana gen. nov. shares with Neorhynchoplax the same general third maxilliped form, the basal part of the antenna is also positioned between the base of the ocular peduncle and antennular fossa, the ambulatory dactylus has at least one subterminal spine, and the G1 structure is similar in general shape. Its carapace form is also very close, possessing a distinct tooth on the lateral surfaces of the carapace ( Fig. 3 View FIGURE 3 A, B). Aletheiana gen. nov., however, differs markedly from most Neorhynchoplax species in that the merus of the third maxilliped is more elongated and the ischium is short ( Fig. 3 View FIGURE 3 E) (versus with both merus and ischium more quadrate and the latter article longer in most Neorhynchoplax , e.g., Ng & Chuang 1996: fig. 27C); the median part of the posterior margin of the epistome consists of two broad lobes separated by a shallow depression ( Fig. 3 View FIGURE 3 C) (median part of the posterior margin of the epistome formed by a distinctly triangular plate in Neorhynchoplax , e.g., Naruse et al. 2008b: fig. 6b; Ng et al. 2011: fig. 2C); and the male abdomen is more elongated and slender, with the telson linguiform and long ( Fig. 3 View FIGURE 3 F–H) (proportionately broader and the telson is short in Neorhynchoplax , e.g., Ng & Chuang 1996: fig. 27F; Ng et al. 2011: fig. 5A). Most Neorhynchoplax species have three distinct rostral lobes with the median one longest; only in two species, N. nasalis ( Kemp, 1917) ( India) , and N. minima ( Lucas & Davie, 1982) ( Australia) are the lateral lobes absent. In these two species, however, the median rostral lobe is very elongated and marginal in position (cf. Kemp 1917: fig. 12; Lucas & Davie 1982: fig. 2a). In Aletheiana gen. nov., the median rostral lobe is short and submarginal ( Fig. 3 View FIGURE 3 A–C).
Aletheiana View in CoL gen. nov. has the same third maxilliped structure as Sulaplax View in CoL , except that its merus is relatively less elongated ( Fig. 3 View FIGURE 3 E) (longer in Sulaplax, Naruse et al. 2008b View in CoL : fig. 4c). In Aletheiana View in CoL gen. nov., the median rostral lobe is still clearly visible from dorsal view, even though it is relatively small and positioned subventrally ( Fig. 3 View FIGURE 3 A– C) (completely absent in Sulaplax, Naruse et al. 2008b View in CoL : fig. 4a, b); the base of the antenna is positioned below the base of the ocular peduncle and antennular fossa ( Fig. 3 View FIGURE 3 C) (positioned more laterally beyond the antennular fossa and base of the ocular peduncle in Sulaplax, Naruse et al. 2008b View in CoL : fig. 4b); the cutting edges of the chela are armed with distinct teeth at least along the proximal half ( Fig. 4 View FIGURE 4 A) (cutting edges unarmed along most of the length except for some proximal denticles in Sulaplax, Naruse et al. 2008b View in CoL : fig. 5b, c); the ambulatory dactylus is armed with at least one subterminal spine ( Fig. 4 View FIGURE 4 B–I) (versus completely smooth and unarmed in Sulaplax, Naruse et al. 2008b View in CoL : fig. 5a), the male abdomen is more elongated and slender with the telson linguiform and long ( Fig. 3 View FIGURE 3 F–H) (proportionately broader and the telson is short in Sulaplax, Naruse et al. 2008b View in CoL : fig. 5d).
The structure of the antennule, and proportions of the merus of the third maxilliped and male abdomen require comment. The antennular fossa of some hymenosomatids is atypical among brachyurans in that it is shallow, and the basal article does not fill the entire space. The antennular articles clearly cannot completely fold into the fossa as with most crabs. Ng (1991: 62) observed that the fossa for Cancrocaeca View in CoL was very shallow and the basal article was small and does not fill it, although he incorrectly observed it was was fused to the cephalothorax. Its basal antennular article is actually short and quadrate but free ( Ng 1991: fig. 2B; Naruse et al. 2008b: fig. 1b). Naruse et al. (2008 b: 24, 31) noted that the basal articles of Sulaplax View in CoL and Guaplax View in CoL are “swollen” but this is not so – the fossa is just shallow, poorly demarcated and the articles are actually short and quadrate (cf. Naruse et al. 2008b: figs. 4b, 7b). As for the third maxilliped, in most Neorhynchoplax View in CoL species, it is quadrate and not elongated (e.g., Ng & Chuang 1996: fig. 27C). Naruse et al. (2008 b: 26–27, fig. 4c) argued that this was a character which separated Neorhynchoplax View in CoL from Sulaplax View in CoL , the structure of the latter genus being elongated and ovate. In at least one species of Neorhynchoplax View in CoL ( N. patnahi Ng, Nesemann & Sharma, 2011 View in CoL , from India), however, the merus of the third maxilliped resembles that of Sulaplax View in CoL (cf. Ng et al. 2011: fig. 3B). In all other characters, however, N. patnahi View in CoL , is a typical member of Neorhynchoplax View in CoL . This is similar for other recently described species such as N. euryrostris Davie & Richer View in CoL de Forges, 1996, and N. elongata Rahayu & Ng, 2004 View in CoL (cf. Davie & Richer de Forges 1996; Rahayu & Ng 2004). As such, this part of the maxilliped may not be that valuable as a generic character. For the male abdomen, somite 5 is usually completely fused with somite 4 (e.g., see Ng & Chuang 1996: fig. 27F), with the sutures absent. In some cases, the suture between somites 4 and 5 is still visible but the two somites are barely or not movable. Ng (1988: 275, fig. 1e) reported that the male abdominal somites 4 and 5 for N. mangalis Ng, 1988 View in CoL , were separate, but Ng & Chuang (1996: 59) noted that although smaller male specimens have somite 5 separated from somite 4 by a suture, they are barely movable, with the suture becoming faint and the two somites becoming completely immobile in large individuals ( Ng & Chuang 1996: fig. 25E). The same is observed here for Aletheiana tenella View in CoL gen. et sp. nov., with the suture between somites 4 and 5 visible but shallow, and the two structures are effectively immobile ( Fig. 3 View FIGURE 3 G).
The G1 structures of Sulaplax View in CoL and Aletheiana View in CoL gen. nov. are remarkably similar in form, and both have the distinctive median twisting with long setae lining the distal half ( Fig. 4 View FIGURE 4 J–L; Naruse et al. 2008b: fig. 5e, f). They also share the same structure of the posterior margin of the epistome ( Fig. 3 View FIGURE 3 C; Naruse et al. 2008b: fig. 4b). They are likely to be sister taxa, especially since both are from Sulawesi. Their locations, are however, far apart. The type locality of Aletheiana tenella View in CoL gen. et sp. nov. is at the northeast of the island, while Muna Island (type locality of Sulaplax ensifer View in CoL ) is some 400 km to the southeast of Sulawesi.
Guaplax Naruse, Ng & Guinot, 2008 View in CoL (type and only species Guaplax denticulata Naruse, Ng & Guinot, 2008 View in CoL ) from southern Borneo is close to Sulaplax View in CoL and Neorhynchoplax View in CoL . Guaplax View in CoL shares with Sulaplax View in CoL the reduced and depigmented eyes which are immobile; and with Neorhynchoplax View in CoL characters of the carapace, third maxillipeds and male abdomen. The differences of the new genus with Sulaplax View in CoL and Neorhynchoplax View in CoL also apply for Guaplax View in CoL . Guaplax View in CoL also differs from Aletheiana View in CoL gen. nov. (as well as Sulaplax View in CoL and Neorhynchoplax View in CoL ) in that it has a trilobate rostrum, the base of the antenna is positioned directly below the base of the ocular peduncle, the distal outer angle of male abdominal somite 1 being lobiform, and the G2 has a dilated lobiform base ( Naruse et al. 2008b: 32).
Samarplax Husana, Tan & Kase, 2011 (type and only species Samarplax principe Husana, Tan & Kase, 2011) from Samar, Philippines, is another troglobitic species similar to Aletheiana View in CoL gen. nov.. This unusual genus shares with Neorhynchoplax View in CoL , Sulaplax View in CoL and Guaplax View in CoL the same kind of third maxilliped and male abdomen; and the nearcomplete absence of a rostrum, structure of the third maxilliped dactylus, position of the base of the antenna, structure of the posterior margin of the epistome and possession of unarmed ambulatory dactyli are similar to the conditions in Sulaplax View in CoL (cf. Husana et al. 2011: figs. 4a–e, 5a, 7). Samarplax is unique, however, in having unarmed cutting edges of the cheliped fingers covered with short brush-like setae ( Husana et al. 2011: fig. 8). Like Sulaplax, Aletheiana View in CoL gen. nov. differs markedly from Samarplax in the position of the antenna, possessing a long third maxilliped dactylus, the ischium of the third maxilliped does not have a projection on the inner distal margin, and the shape of the male abdomen is different (longer and narrower) with the telson elongated and linguiform.
As to freshwater taxa, there are a large number of hymenosomatids which have been reported from the Old World, mostly from genera like Amarinus Lucas, 1980 View in CoL , Odiomaris Ng & Richer View in CoL de Forges, 1996, Hymenicoides Kemp, 1917 View in CoL , Limnopilos Chuang & Ng, 1991 View in CoL , and Cancrocaeca Ng, 1991 View in CoL . Members of these genera, however, have very distinctive broad male abdomens and very short and stout G1 structures in which the distal part may have lobes and projections (see Kemp 1917; Lucas 1980; Chuang & Ng 1991; Ng 1991; Ng & Richer de Forges 1996; Naruse et al. 2008b; Guinot 2011a, b). Sulaplax View in CoL , Guaplax View in CoL and Samarplax are both monotypic freshwater genera ( Naruse et al. 2008b; Husana et al. 2011). As for Neorhynchoplax View in CoL , most species occur in estuarine and mangrove habitats, usually in intertidal waters. Five species, however, are known from freshwater habitats: N. introversa ( Kemp, 1917) ( China) View in CoL ; N. kempi ( Chopra & Das, 1930) ( Iraq) View in CoL ; N. inermis Takeda & Miyake, 1971 ( Palau) View in CoL ; N. dentata Ng, 1995 ( Sarawak) View in CoL ; N. patnahi Ng, Nesemann & Sharma, 2011 ( India) View in CoL ; and N. prima Ng & Chuang, 1996 ( Pulau Bintan) View in CoL (cf. Chopra & Das 1930; Shen 1932; Takeda & Miyake 1971; Abele 1972; Chuang & Ng 1994; Ng & Chuang 1996; Ng et al. 2011).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
Aletheiana
| Ng, Peter K. L. & Lukhaup, Christian 2015 |
N. patnahi
| Ng, Nesemann & Sharma 2011 |
N. patnahi
| Ng, Nesemann & Sharma 2011 |
Sulaplax
| Naruse et al. 2008 |
Sulaplax
| Naruse et al. 2008 |
Sulaplax
| Naruse et al. 2008 |
Sulaplax
| Naruse et al. 2008 |
Sulaplax
| Naruse et al. 2008 |
Sulaplax
| Naruse et al. 2008 |
Guaplax
| Naruse, Ng & Guinot 2008 |
Guaplax denticulata
| Naruse, Ng & Guinot 2008 |
N. elongata
| Rahayu & Ng 2004 |
N. dentata
| Ng 1995 |
Limnopilos
| Chuang & Ng 1991 |
Cancrocaeca
| Ng 1991 |
N. mangalis
| Ng 1988 |
Amarinus
| Lucas 1980 |
N. inermis
| Takeda & Miyake 1971 |
N. kempi (
| Chopra & Das 1930 |
Hymenicoides
| Kemp 1917 |
N. introversa (
| Kemp 1917 |