Anolis robinsoni, Chaves & Ryan & Bolaños & Márquez & Köhler & Poe, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5319.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:065A967F-2832-4BDF-890A-2EA852CACD74 |
|
DOI |
https://doi.org/10.5281/zenodo.8184545 |
|
persistent identifier |
https://treatment.plazi.org/id/09619A08-FFA9-190E-8FEC-F8D09C30FE81 |
|
treatment provided by |
Plazi |
|
scientific name |
Anolis robinsoni |
| status |
sp. nov. |
Anolis robinsoni View in CoL , new species
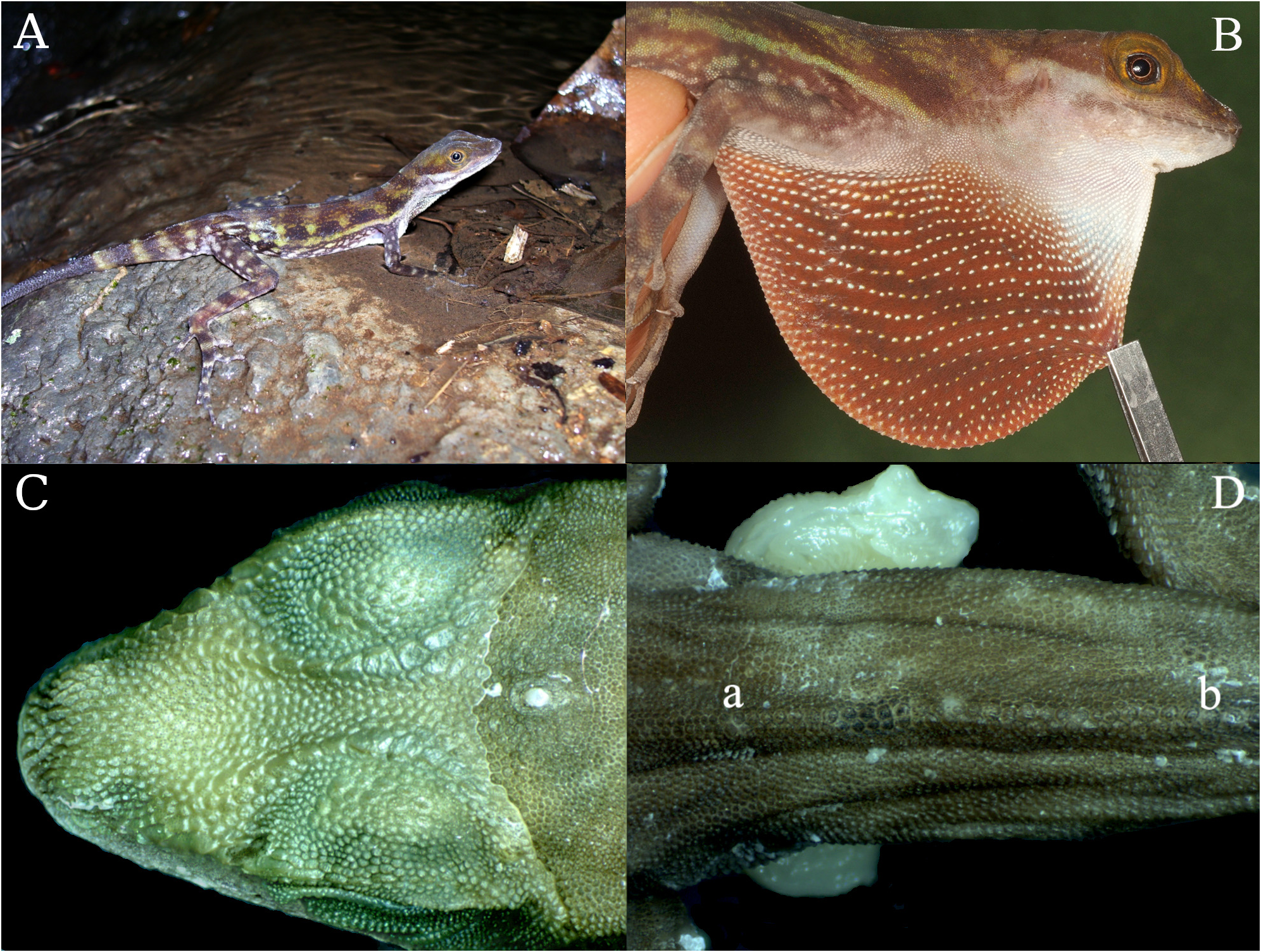
Holotype. UCR 2463 ( Fig. 4 View FIGURE 4 ) collected 15 March 1969 by Oscar Blanco , an adult male from Palma stream bridge 5.1 km south of Santa Marta de Santiago de Puriscal, 9.79230 N, - 84.39530 W, ca. 800 masl, San José Province, Costa Rica. GoogleMaps
Paratypes ( all from Costa Rica: San José Province). Adult males: SMF 92437 About SMF (collected 27 March 2010 by Gunther Köhler) from Zona Protectora El Rodeo ( Universidad para la Paz), 9.90392 N, - 84.28169 W, ca. 825 masl GoogleMaps ; SMF 92438 About SMF (collected 27 March 2010 by Gunther Köhler) from Zona Protectora El Rodeo ( Universidad para la Paz), 9.90355 N, - 84.28192 W, ca. 780 masl GoogleMaps ; UCR 2464 (same data of the holotype); UCR 16048 (collected 28 October 2001 by Mason J Ryan, Robert Puschendorf and Brian Kubicki) from the west slope of the Cerros de Escazú, Río Jaris ( 9.88460 N, - 84.27650 W, 500 ca. masl), close to the Zona Protectora El Rodeo ( Universidad para la Paz) GoogleMaps ; MCZ R-186162 (collected 27 December 2007 by Steve Poe and Mason J. Ryan) from Quebrada La Palma in Alto Palma de Puriscal ( 9.78890 N, - 84.39440 W, ca. 1000 masl). Adult females GoogleMaps : SMF 92439 About SMF , 92450–51 About SMF (collected 27 March 2010 by Gunther Köhler) from Zona Protectora El Rodeo ( Universidad para la Paz), 9.90364 N, - 84.28182 W, ca. 820 masl GoogleMaps ; UCR 2558 (collected 15 March 1969 by Oscar Blanco and Douglas Robinson) and MCZ R-186161 (collected 27 December 2007 by Steve Poe and Mason J. Ryan) both of them from Quebrada La Palma in Alto Palma de Puriscal ( 9.78890 N, - 84.39440 W, ca 1000 masl) GoogleMaps .
Diagnosis. Anolis robinsoni sp. nov. is a semiaquatic anole that is distinguished from all other Anolis of Central America by male dewlap coloration (chocolate brown with ill-defined brick red horizontal streaks, Fig. 4B View FIGURE 4 ). The only other species with which A. robinsoni sp. nov. might be confused in the field are the other Middle American semiaquatic anoles A. aquaticus , A. lionotus , A. poecilopus , and A. barkeri . Anolis lionotus and A. poecilopus are easily distinguished from A. robinsoni sp. nov. by male dewlap color (dull orange-yellow in these species, chocolate brown with ill-defined brick red horizontal streaks in A. robinsoni sp. nov.), presence of enlarged postcloacal scales in most males (absent in A. robinsoni sp. nov.), and presence of a longitudinal zone of 10–24 enlarged middorsal scales (0–3 scales enlarged in A. robinsoni sp. nov.). Anolis aquaticus may be distinguished from A. robinsoni sp. nov. by male dewlap color (orange-red with yellow in A. aquaticus , Fig. 5A View FIGURE 5 ) and possession of larger dorsal scales of the head and body (e.g., 15–20 scales across the snout between second canthals in A. robinsoni sp. nov. ( Fig. 4C View FIGURE 4 ), 7–14 in A. aquaticus ; Fig. 5B View FIGURE 5 , Table 1 View TABLE 1 ) and the size of the middorsal caudal row scales relative to adjacent scales (no more than two times in A. robinsoni sp. nov. ( Fig. 4D View FIGURE 4 ), more than three times in A. aquaticus ( Fig 5C View FIGURE 5 ). Anolis barkeri is most easily distinguished from A. robinsoni sp. nov. by its larger size (maximum SVL 91 mm in A. barkeri , 74 mm in A. robinsoni sp. nov.), lack of distinctly expanded toe pads (ratio width of expanded pads/width of distal phalanx 1.8–2.2 in A. robinsoni sp. nov. versus 1.4–1.6 in A. barkeri ), and presence of a double row of middorsal caudal scales (single row in A. robinsoni sp. nov.).
Description of the holotype ( Fig. 4 View FIGURE 4 ). Adult male as indicated by dewlap and everted hemipenes; SVL 63.9 mm; HL 17.5 mm; HL/SVL 0.28; HW 10.1 mm; EOH 2.36 mm; IL 1.18 mm; IL/EOH 0.50; SLS-I 0.18 mm mm; AGL 23.1 mm; FL 16.6 mm; T4L 15.1 mm; T4W 1.42 mm; TL 10.4 mm, tail complete; TL/SVL 0.38; T4L 15.1 mm and T4W 1.42 mm. 18 scales across the snout between second canthals; two scales separate the nasal opening from rostral scale; nine postrostral scales between supralabials; nine supralabial scales from rostral scale to level of middle of eye; six scales separating supraorbital semicircles; one rows of scales separate subocular scales from supralabials; two slightly elongate superciliary scale; eight scales separating interparietal scale from supraorbital semicircles; 12 enlarged scales in supraocular disk; 11 loreal scales in column just anterior to eye; 11 postmental scales posteriorly in contact with mental scale between infralabials; 16 scales in the loreal region; 19 rows of single scales in the dewlap (10–42 scales per row, rows somewhat irregular); 2 middorsal scale rows slightly larger than adjacent scales; 16 dorsal scales in 5% of SVL; 12 ventral scales in 5% of SVL; 220 scales around midbody; 23 expanded lamellae under fourth toe; 26 small middorsal caudal scales before the first large caudal scale ( Fig. 4D View FIGURE 4 ). Preoccipital, enlarged infralabial, tail crest, middorsal, and postcloacal scales absent; supraocular disc presents small scales. Frontal region of head concave; scales in supraocular disc unicarinate; rostral scale with weak cleft and overlaps mental scale; ear opening vertically ovoid; dorsal edge of ear lacks ornamentation; mental scale partially divided posteriorly and with its posterior edge convex; dewlap extends well on to chest with anterior insertion at level of anterior portion of the eye and without protruding scales at distal edge; dorsal scales small, keeled with two middorsal scale rows slightly larger than adjacent scales; ventral scales in diagonal rows, keeled and larger than dorsal scales; lateral scales homogeneous; tail is compressed and triangular in cross-section with the base taller than wide; middorsal caudal scales in single row and its size is less than three times than the adjacent scales ( Fig. 4D View FIGURE 4 ); the longest toe of adpressed limb reaches anterior to eye and, supradigital scales keeled and multicarinate.
Color in life (male based on field notes and color photographs; Fig. 4A and 4B View FIGURE 4 ). The dewlap of males in life is chocolate brown with lighter orange-brown bars located centrally. The dorsal ground color is olive to chestnut brown with transverse olive–green bars across dorsum down flanks extending to tail. The head is uniformly dark or light brown. A cream-colored line extends posteriorly from the labial scales down the side of the body. Scattered small greenish spots are present on the dorsum. Scales on the tip of the snout with black pigmentation. The dorsum and tail are marked with olive–green transverse bars and the limbs marked with pale green to yellowish transverse bars with small punctuations.
Variation. Anolis robinsoni sp. nov. showed variation in the SVL of reproductive males (58.6 ± 11.0, 42.1–73.8 mm). Table 1 View TABLE 1 shows the variation in lepidosis. The gular region of females is white with brown streaks. Females have a small brown to black dewlap. Transverse, dorsal olive-green bands of males varied between 7–8, with 10–12 tail bands; females had 7–8 olive–green dorsal transverse bars with 9–11 tail bands. In life, the dorsal coloration of both sexes may vary with transverse bands darker when cold or stressed (e.g., when held in hand).
Hemipenis morphology. The almost completely everted hemipenis of SMF 92438 ( Fig. 7A View FIGURE 7 ) is a stout bilobed organ. The sulcus spermaticus is bordered by well-developed sulcal lips opening into two broad concave areas, one on each lobe. A small asulcate ridge is present. The apex is strongly calyculate, truncus with transverse folds.
Etymology. The specific epithet is a patronym in honor of the late Douglas C. Robinson, who contributed enormously to the herpetological knowledge of Costa Rica through extensive field collections and by inspiring a generation of Costa Rican biologists. He received his Ph.D. from Texas A&M University in 1968 and studied the Mexican semiaquatic anole, Anolis barkeri .
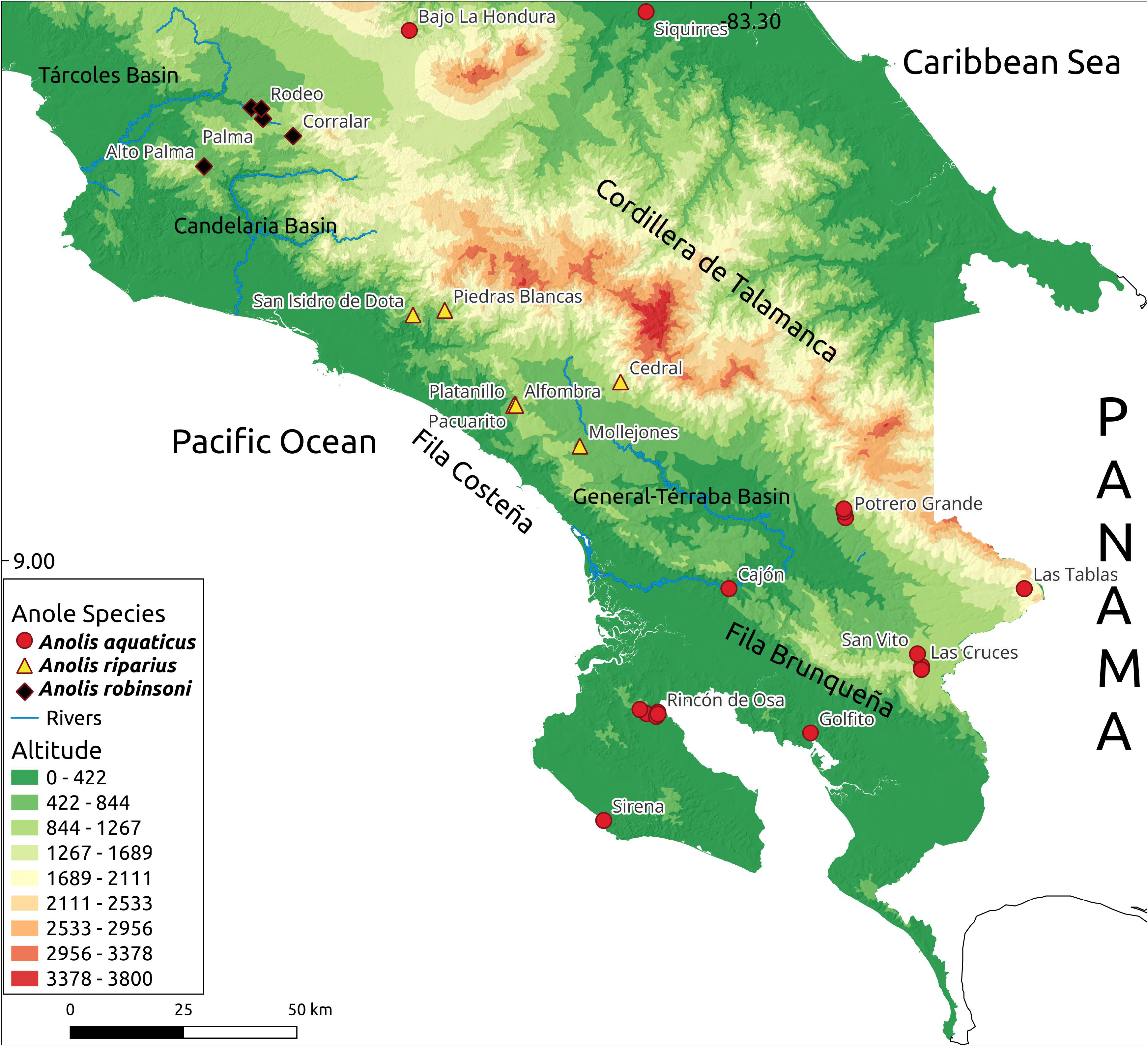
Distribution. Anolis robinsoni sp. nov. is known from the riparian gallery forests of the Río Candelaria basin ( Fig. 1 View FIGURE 1 ), the foothills of the Montañas de Turrubares in the northwestern section of the Cordillera de Talamanca, and Río Jaris along the western border of the Zona Protectora El Rodeo. These areas are within the Tropical Wet and Humid Premontane forest life zones ( Holdridge 1967). Anolis robinsoni sp. nov. has been found at elevations between 500 and 1100 masl.
Ecology. Most of the following observations are from Márquez–Baltán (1994) and Márquez et al. (2005) from Quebrada La Palma. During the day this species is found on bare rocks and boulders along streams with dense canopy cover. At night it is found sleeping on moss and vegetation on boulders near the splash zones of small and medium waterfalls. Both sexes and juveniles were found no more than two meters from the edge of a stream. The operational sex ratio is approximately equal proportions of males to females ( Márquez et al. 2005), and males share territories with one to three females. The estimated population density varied from 100 to 200 individuals per hectare and population size fluctuated monthly with a peak during the dry season.
Reproduction occurs throughout the year with a lull in the wet season (May to November). Females lay 1 egg per clutch in cracks and interstitial spaces of boulders in streams. Up to 6 eggs have been found together suggesting that this species lays eggs in communal nest sites. Incubation times ranged between 75–82 days, and hatchlings are approximately 25 mm SVL ( Márquez et al. 2005).
| MCZ |
Museum of Comparative Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.