Zanna Kirkaldy, 1902
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4338.2.10 |
|
publication LSID |
lsid:zoobank.org:pub:48170103-AAEC-4E65-BCF9-27014C2CD079 |
|
DOI |
https://doi.org/10.5281/zenodo.6052748 |
|
persistent identifier |
https://treatment.plazi.org/id/097487B5-FFDA-FFDF-FF08-7F3ACF8391A7 |
|
treatment provided by |
Plazi |
|
scientific name |
Zanna Kirkaldy, 1902 |
| status |
|
Genus Zanna Kirkaldy, 1902 View in CoL View at ENA
Pyrops Amyot et Audinet-Serville, 1843: 491 View in CoL ; Atkinson, 1885: 139; Distant, 1906: 179; Li, 1987: 330. Zanna Kirkaldy, 1902: 47 View in CoL ; Melichar, 1903: 13; Metcalf, 1947: 246; Lallemand, 1963: 90; Chou et al., 1985: 113; Nagai & Porion, 1996: 27; Hua, 2000: 96. Type species: Fulgora tenebrosa Fabricius, 1775 View in CoL . Replacement name for Pyrops Amyot View in CoL et Audinet- Serville, 1843 nec Pyrops Spinola, 1839 View in CoL .
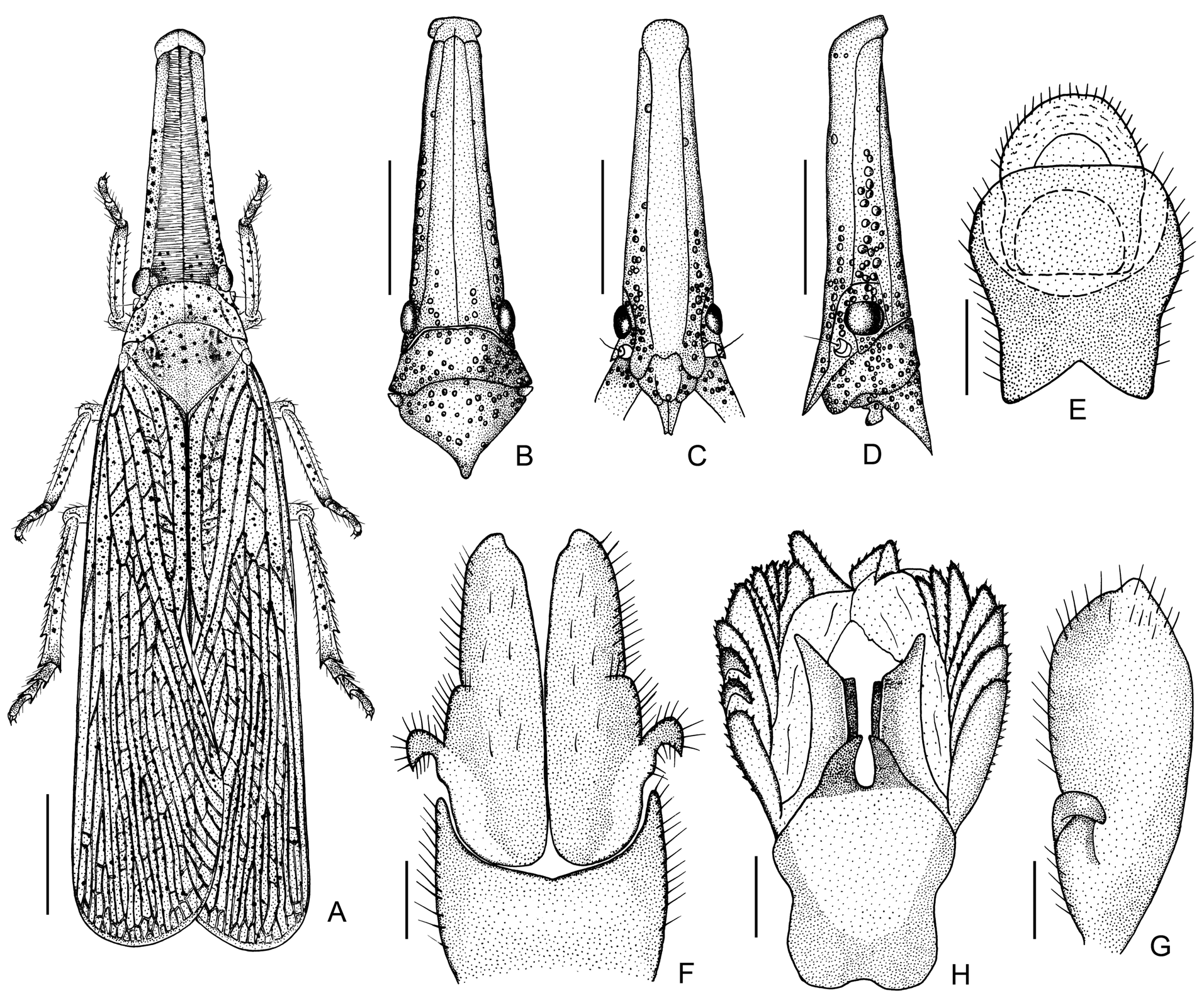
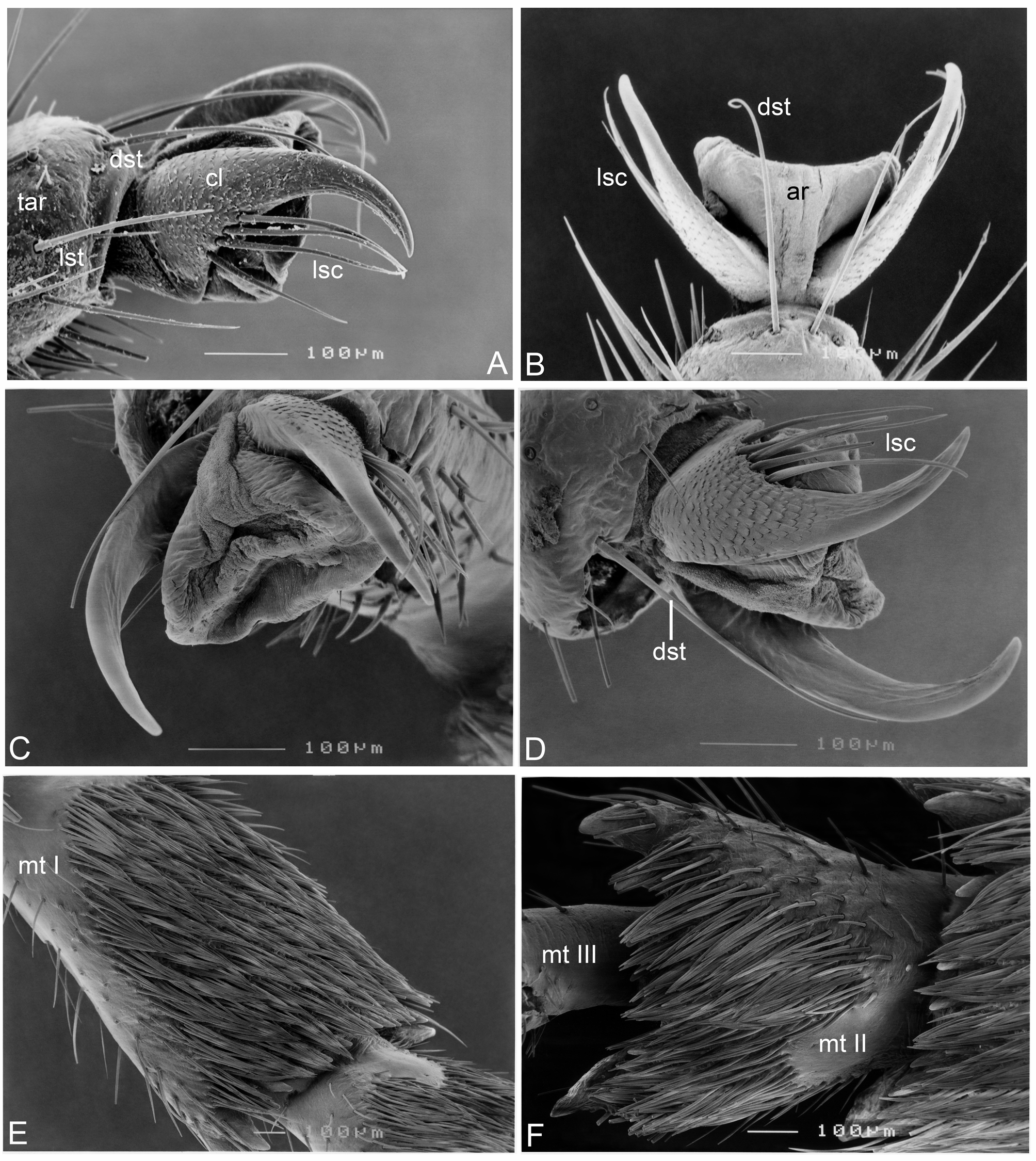
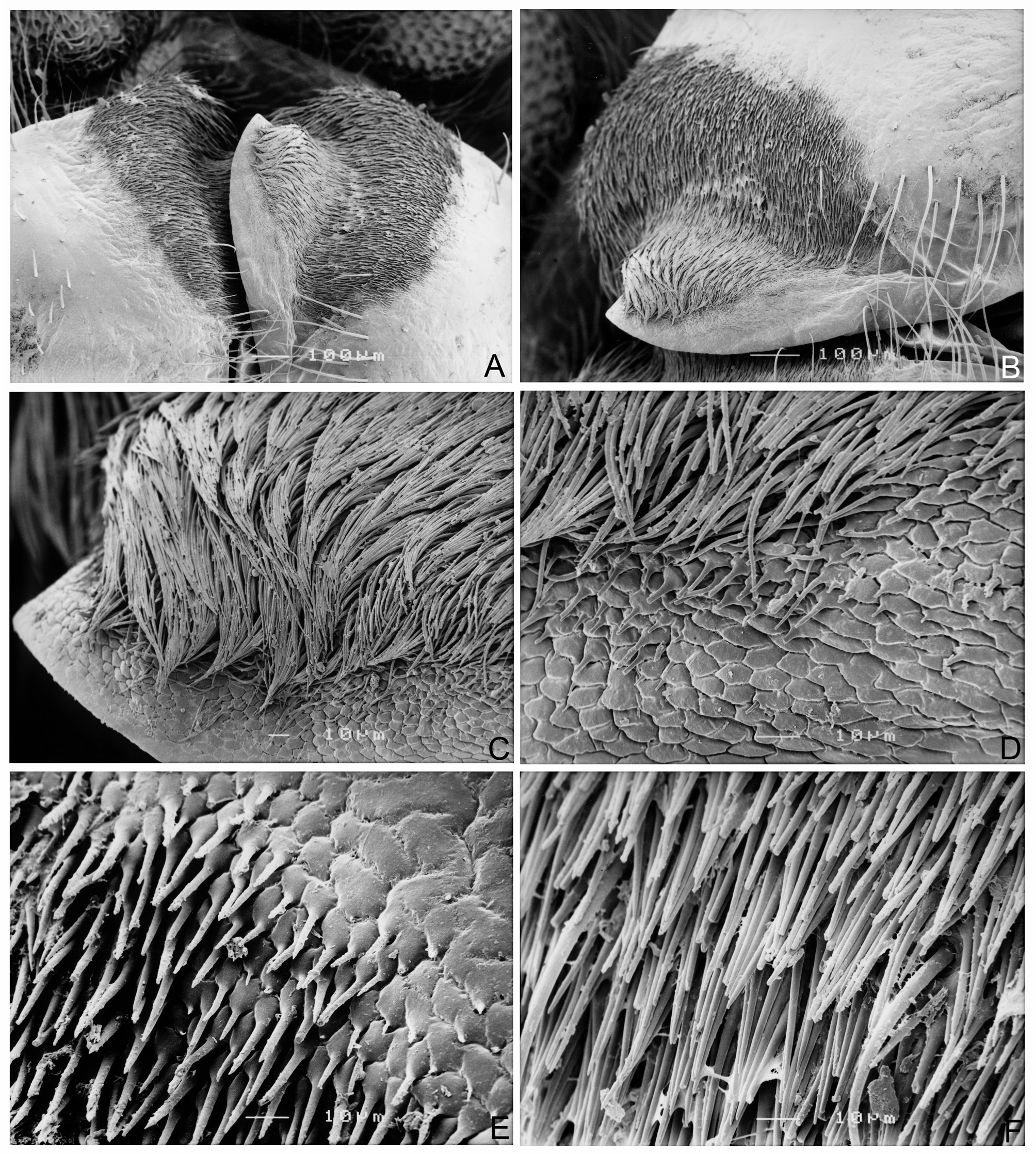
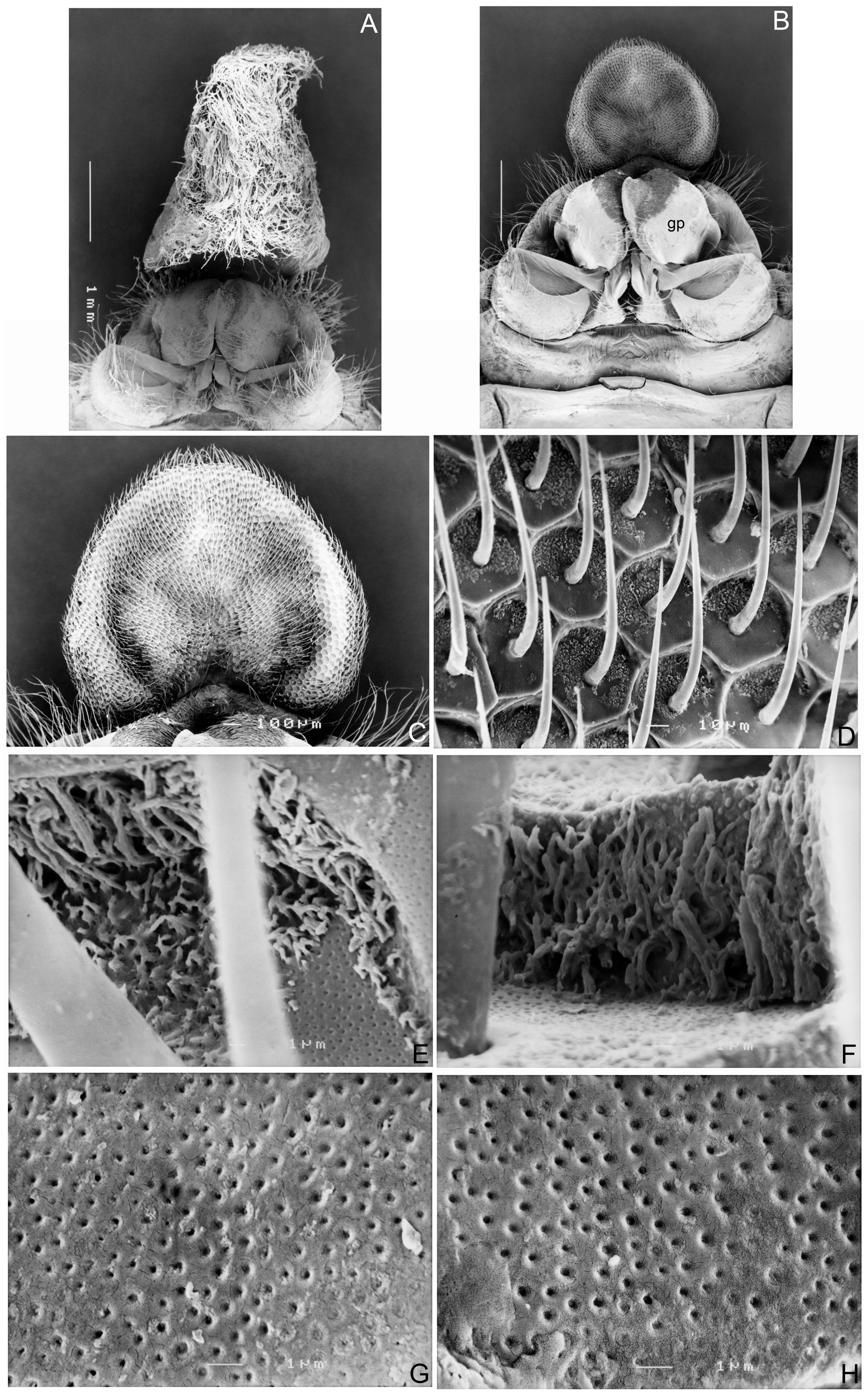
Revised generic diagnosis. Members of Zanna can be distinguished from other Fulgoridae genera by the combination of the following characters: body distinctly narrow, slender and elongate, usually pale brownish ochraceous, without colorful markings, usually thickly and prominently spotted in black ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 , 3A–D View FIGURE 3 ); head distinctly produced into a very long cephalic process, which is longer than abdomen and more than twice as long as pronotum and mesonotum combined; cephalic process relatively robust, hexagonal, gently narrowing from base to apex, somewhat upturned at apex in lateral view ( Figs 1 View FIGURE 1 , 2A–D View FIGURE 2 , 3A–D View FIGURE 3 ); vertex very elongate, lateral margins weakly ridged with carinae being slightly zigzagged, with faint median carina ( Figs 1 View FIGURE 1 , 2A, 2B View FIGURE 2 , 3A, 3B View FIGURE 3 ); frons distinctly elongate, with lateral margins weakly ridged with carinae being slightly zigzagged, with slightly zigzagged submedian carinae, without median carina ( Figs 2C View FIGURE 2 , 3C View FIGURE 3 ); antennae with pedicle relatively short, subbulbose, fully covered with sensory plaque organs over entire surface (Figs 4, 5A–D); forewings narrow and elongate, almost entirely reticulate, inner marginal areas overlapping at tips; hind tibiae with 5–7 lateral spurs; metatarsomes I and II covered with a pad of dense setae ventrally ( Figs 6E, 6F View FIGURE 6 ); gonoplac in female adults with basal inner area strongly and densely pilose ( Figs 7 View FIGURE 7 , 8A, 8B View FIGURE 8 ); anal tube in female adults expanded, nearly cardiform in ventral aspect ( Figs 8B, 8C View FIGURE 8 ), densely covered with long, whitish wax threads ventrally ( Fig. 8A View FIGURE 8 ), its ventral surface covered with numerous fine hexagonal wax gland units ( Fig. 8D View FIGURE 8 ), each hexagonal unit bearing a long seta coming from center ( Fig. 8B–D View FIGURE 8 ) and numerous very tiny wax gland pores on surface ( Fig. 8E–H View FIGURE 8 ).
Monophyly of Zanna . Currently there are about 143 described genera of Fulgoridae ( Bourgoin 2017) , however, the monophyly of most genera has not been examined cladistically. The wax-secreting plates in the adults of most fulgorid species are located on the abdominal tergites VI–VIII ( O’Brien & Wilson 1985). However, the anal tube in the female adults of the Zanna species is expanded and nearly cardiform in ventral aspect and the wax gland pores are located on the ventral surface of the modified anal tube (see description under Z. robusticephalica sp. nov. below). The very similar wax gland plates are also found in some species of the other fulgoroid families, namely Flatidae , Lophopidae , Ricaniidae and Nogodinidae ( Lucchi & Mazzoni 2004; Liang, unpublished data), however, these similarities seem to be the results of parallel developments. In addition, the prolongation of the head into elongate and hexagonal cephalic process ( Figs 1 View FIGURE 1 , 2A–D View FIGURE 2 , 3A–D View FIGURE 3 ), forewings with inner marginal areas overlapping at tips ( Figs 1 View FIGURE 1 , 2F View FIGURE 2 , 3A View FIGURE 3 ); gonoplac in female adults with basal inner area being strongly and densely pilose ( Figs 7 View FIGURE 7 , 8A, 8B View FIGURE 8 ) and the metatarsomeres I and II being covered with a pad of dense setae ventrally ( Figs 6E, 6F View FIGURE 6 ) are found only in Zanna species. These distinct characters appear to be good autapomorphies of Zanna that support the monophyly of the genus.
Biology. In common with most planthopper groups, few biological data are available for species of Zanna . Gade (1985) reported that Z. madagascariensis in Madagascar feeds Lima bean and related plants and that the adults of Z. madagascariensis are known as sakandry, and are consumed by the rural people of Madagascar (van der Heyden 2014). Chou et al. (1985) and Hua (2000) reported that Z. chinensis can infest Glycine max (Linn.) Merr. (soybean) and Cocos nucifera L. (coconut palm) in China. Li (1987) recorded the occurrence of Z. chinensis as Pyrops chinensis in Guizhou of southwest China and reported that in Guizhou the adults of this species can be attracted under light in early May and the nymphs can be found in late October infesting soybeans.
Distribution. Oriental region ( India (northern part), Sri Lanka, Sikkim, Bengal, Nepal, China (southern and southwestern parts), Vietnam, Thailand, Malaysia (Malay Peninsula, Borneo), Singapore, Indonesia (Java, Sumatra), Afrotropical region.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Zanna Kirkaldy, 1902
| Liang, Ai-Ping 2017 |
Pyrops
| Hua 2000: 96 |
| Nagai 1996: 27 |
| Li 1987: 330 |
| Chou 1985: 113 |
| Lallemand 1963: 90 |
| Metcalf 1947: 246 |
| Distant 1906: 179 |
| Melichar 1903: 13 |
| Kirkaldy 1902: 47 |
| Atkinson 1885: 139 |
| Amyot 1843: 491 |