Eriocheir ogasawaraensis Komai, 2006
|
publication ID |
https://doi.org/10.5281/zenodo.2645512 |
|
DOI |
https://doi.org/10.5281/zenodo.6253848 |
|
persistent identifier |
https://treatment.plazi.org/id/0D298791-D264-FFA1-FE88-4F4E0F4DFD83 |
|
treatment provided by |
Plazi |
|
scientific name |
Eriocheir ogasawaraensis Komai |
| status |
sp. nov. |
Eriocheir ogasawaraensis Komai View in CoL , n. sp.
( Figs. 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 7 , 9 View FIGURE 9 )
Eriocheir japonicus View in CoL . — Miyake, 1970: 289; Takeda & Miyake, 1976: 112; Muraoka, 1998: 52 [not Eriocheir japonica de Haan, 1835 View in CoL ]; Miyake, 1983: 174 (in part). “ Mokuzugani. ” — Yamamoto, 2004: 16, figs. 1–3; Kobayashi, 2005: 17 –22, figs. 1, 3, 6, 8, 9.
Material examined
HOLOTYPE. CBMZC 8503, male (76.2 X 90.2 mm), rocky beach near Yatsusegawa river mouth, Chichijima I., Ogasawara Is, subtidal, 16 March 2004, trap, coll. S. Kobayashi.
PARATYPES. CBMZC 8504, 1 female (81.0 X 95.9 mm), same data as holotype; CBMZC 8571, 1 male (65.7 X 76.0 mm), 2 nonovigerous females (50.7 X 60.0 mm, 74.7 X 87.6 mm), 1 ovigerous female (72.7 X 86.1 mm), Yatsusegawa river , April 2005, leg. S. Yoneyama ; NTOU, 1 male (60.5 X 70.5 mm), 1 ovigerous female (74.1 X 86.1 mm), same data; NSMTCr 16818, 1 male (61.5 X 71.7 mm), 1 ovigerous female (61.4 X 71.5 mm,), same data; ZRC 2005.0146 View Materials , 1 female (70.3 X 80.0 mm,), same data; CBMZC 8572, 2 males (35.0 X 41.0 mm, 46.3 X 54.2 mm), 3 young females (36.0 X 42.5 mm, 41.0 X 49.0 mm, 44.5 X 50.8 mm), 1 nonovigerous female (68.8 X 80.7 mm), Okumura River , Chichijima I., 1 km from river mouth, 13 July 2005, hand, coll. J. Kimura & F. Yumura ; ZRC 2005.0145 View Materials , 1 male (46.0 X 53.2 mm), same data; KMNH, 1 young female (38.7 X 44.3 mm), Otoutojima I., 16 September 1996, coll. H. Karube ; NSMTCr 16819, 1 male (76.6 X 88.0 mm), Hatsune , Chichijima I., stream, 11 December 2005, coll. K. Satake.
Nontype (all collected by T. Sasaki & M. Fujita). CBMZC 8573, 1 male (81.9 X 94.8 mm), Akatodai, Chichijima I., December 1998; CBMZC 8574, 1 male (49.0 X 56.4 mm), 2 females (15.5 X 17.5, 30.6 X 35.2 mm), north of Sakaiura Beach , Chichijima I., January 1998 ; CBMZC 8575, 1 male (87.7 X 104.7 mm), upper stream of Shigure Dam, Chichijima I., February 1998; CBMZC 8576, 6 males (21.2 X 23.9, 24.0 X 26.9, 26.8 X 29.7, 27.2 X 31.6, 36.8 X 41.6, 37.7 X 42.2 mm), east of Tatsumi , Chichijima I., March 1998 ; CBMZC 8577, 1 male (73.4 X 86.0 mm), river mouth at Tatsumi Beach, Chichijima I., April 1998; CBMZC 8578, 1 male (35.2 X 40.4 mm), 1 female (64.3 X 75.3 mm), Nishiura Beach, Hahajima I., May 1998; CBMZC 8579, 1 male (18.2 X 21.3 mm), north of Nishiura Beach , June 1998 ; CBMZC 8580, 1 male (72.3 X 84.8 mm), Tamanagawa river mouth, Anijima I., July 1998 .
Description of adult males
Carapace ( Figs. 1A View FIGURE 1 , 2A, B View FIGURE 2 ) rectangular, greatest width across bases of second ambulatory legs (third pereopods) 1.15–1.20 of carapace length; distance between external orbital teeth 0.48–0.55 of cl; distance between second anterolateral teeth 0.94–0.96 of cl; distance between third anterolateral teeth 1.10–1.12 of cl; overall dorsal surface nearly flat, minutely punctate. Epigastric ridge moderately high, anterior margins bluntly edged; protogastric ridges low, granular, bluntly edged. Hepatic regions slightly depressed posteriorly. Gastrocardiac, cardiobranchial grooves distinct, cardiointestinal groove shallow. Branchial regions slightly swollen. Epibranchial ridge high, granular, sharply edged. Mesobranchial ridge low but sharply delimited, granular, extending obliquely backwards, not reaching lateral margin. Posterolateral ridge distinct, granular.
Frontal width 0.42–0.48 of width between external orbital teeth, 0.26–0.28 of carapace width; frontal margin granular, weakly divided in 4 lobes ( Fig. 2A View FIGURE 2 , 5A), median lobes broadly rounded, lateral lobes exceeding as far as median lobes, much narrower than median lobes, bluntly triangular. Supraorbital margin deeply concave, with small median cleft; orbital cavity with submarginal row of dense, short, dorsal setae; infraorbital ridge short, oblique in anterior view, extending onto inner side of orbit, separated from outer orbital ridge by deep concavity; inner orbital tooth small, acute, vertical mesial margin with row of sharp granules.
Anterolateral margins ( Fig. 2A View FIGURE 2 , 5B) coarsely granular, noticeably convergent anteriorly, each with 3 teeth including external orbital tooth; second and third teeth separated by Vshaped notches. External orbital tooth largest, sharply pointed anteriorly, extending nearly as far as lateral frontal lobe, anterolateral margin strongly divergent backwards, sinuous; mesial margin of external orbital tooth corresponding to outer orbital ridge, nearly vertical with slightly convex edge, fringed with small sharp teeth. Second tooth acute, its lateral margin slightly divergent backwards, straight. Third tooth smaller than second, subacute. Fourth tooth usually absent, or if present, represented by granule slightly larger than other marginal granules. Posterolateral margin finely granular, sinuous with shallow concavity to accommodate coxa of fourth ambulatory leg (fifth pereopod). Posterior carapace margin nearly straight, finely granular.
Anterolateral region inferior to external orbital tooth ( Fig. 2B View FIGURE 2 ) shallowly sulcate, with few granules. Suborbital ridge distinct, coarsely granular, extending mesially to buccal frame. Pterygostomial and lateral surfaces covered with coarse granules ( Fig. 2B View FIGURE 2 , 3A View FIGURE 3 ); pterygostomial grooves bordered by row of small granules. Anteroventral margins at bases of chelipeds denticulate.
Epistome ( Fig. 2B View FIGURE 2 ) generally triangular, with dorsolateral projections upturned, produced into antennal fossa, each ornamented by rounded tubercles; dorsolateral margins sinuous; anterior surface concave, dorsal part raised, bearing median row of granules; in ventral view, ventral margin straight or faintly waved with very low, broad median lobe, bordered by rounded granules (Fig. 5C). Endostomal ridge low, but clearly delineated.
Eyes (Fig. 5A) well developed; ocular peduncle short, inflated basally; dorsal tubercle rudimentary; cornea small, darkly pigmented. Antennular peduncle ( Fig. 2B View FIGURE 2 ) folded obliquely; basal segment triangular in anterior view. Antennal peduncle ( Fig. 2B View FIGURE 2 ) with second segment quadrate in anterior view; third segment broadly touching inner orbital margin; flagellum short, reaching tip of external orbital tooth.
Third maxillipeds (Fig. 5D) moderately broad, leaving rhomboidal median hiatus when closed. Ischium longer than broad, with distinct longitudinal sulcus along elevated mesial margin. Merus slightly shorter than ischium, longer than broad, weakly narrowed at base, lateral margin convex, anterolateral angle slightly produced, broadly rounded; ventral surface slightly elevated in midline and noticeably elevated laterally and mesially. Carpus articulated at middle of concave anterior margin of merus. Dactylus longer than propodus. Exopod slender, not reaching anterior margin of merus, with well developed flagellum.
Chelipeds ( Fig. 1A View FIGURE 1 , 4A View FIGURE 4 ) subequal and similar. Coxa with ventrodistal margin smooth or faintly granular; basal margin articulating with sternum by a toothlike hinge; large, toothlike hinge connecting to basis. Anteroventral and posteroventral margins of ischium denticulated or granulated. Merus moderately stout, prismatic in crosssection; anterodorsal margin with 2 or 3 rows of small tubercles or granules; posterior margin convex, with irregular double row of granules, terminating bluntly in distal end, not overhanging subdistal groove; ventral margin with 2 rows of granules; inner distal margin with row of small granules; posteroventral (outer) surface with several transverse rows of small granules, other surfaces nearly smooth. Carpus quadrate in outer view; outer surface covered with very small granules, and with row of granules along inner margin; inner margin sharply ridged, with 2 rows of granules, with small, acute tooth arising medially on inner margin; distal margin bordered by dense, thick, long setae; inner surface narrow, bluntly ridged on midline, with row of sparse granules or small tubercles. Chela large in adult males, attaining 1.09 of carapace length; surfaces covered with dense, thick, long setae concealing dorsal, outer and inner surfaces of palm and proximal part of fingers; 1.86–2.07 times longer than height; ventral surface of palm and fixed finger and proximoventral part of inner surface of palm naked, ventral surface coarsely granular; weak longitudinal ridge on outer surface adjacent to ventral margin, extending beyond midlength of pollex. Pollex with dorsal surface excavate, with thick setae extending nearly to tip, terminal margin with corneous ridge; cutting edge with 6 large, molarlike teeth decreasing in size distally. Dactylus with several rows of minute pits on outer surface; distal part slightly hollowed, bordered by corneous ridge; cutting edge with 5 or 6 large molarlike teeth decreasing in size distally (distal 1 or 2 occasionally eroded).
Ambulatory legs (second to fifth pereopods) ( Fig. 1A View FIGURE 1 , 4D View FIGURE 4 ) moderately long and slender; second and third legs longer than first and fourth legs; merus of third leg 0.68–0.76 of carapace length, 3.20–3.50 times longer than wide; merus of fourth leg 2.96–3.13 times longer than wide; propodus of fourth leg 2.16–2.48 times longer than wide. Coxae quadrate, unarmed. Basisischium of each leg granular on anterior surface (first and second) or smooth (third and fourth). Anterior margin of each merus distinctly ridged, with 2 rows of small granules, terminating subdistally in small, acute tooth; dorsal surface minutely granular, smooth (first) or with blunt, granular longitudinal ridge adjacent to dorsal margin in proximal half; posterodorsal margin distinctly ridged, granular. Each carpus with dense thick long setae on anterior surface; dorsal surface with 2 longitudinal series of stiff setae on either side of midline (first with posterior row consisting of tufts of short setae); ventral surface with single series of long setae and patch of similar setae in first, single row of tufts of long setae in second, row of sparse tufts of short setae in third, smooth in fourth. Propodus of each leg with dense mat of short pubescence and numerous tufts of relatively longer setae on anterior surface; propodus of first leg with median row of sparse tufts of short setae on dorsal surface and dense mats of short pubescence and tufts of longer setae extending onto ventral surface on posterior surface; propodus of second leg with median row of tufts of short setae and narrow mat of pubescence and tufts of moderately short setae on posterior surface; propodus of third leg with 2 rows of tufts of short setae on dorsal surface (setae on anterior row very short) and tufts of short to moderately short setae on posterior surface; propodus of fourth leg strongly compressed, with numerous scattered tufts of short setae on posterior half on dorsal surface and dense moderately long setae on posterior margin. Dactylus of each leg slightly curved, with 1 setose groove on anterior and posterior surfaces, and 2 setose grooves on dorsal and ventral surfaces, terminating in sharp corneous claw; dactylus of first leg nearly conical, subequal in length to propodus; dactyli of second and third legs similar, slightly compressed, 0.80–0.90 length of propodi; dactylus of fourth leg more compressed than others, subequal in length to propodus.
First gonopod (Fig. 6A, B) relatively slender, 8.1–8.5 times longer than broad, reaching suture separating fourth and fifth thoracic sternite, triangular in cross section; ventral (outer) surface shallowly sulcate medially; lateral surface with distinct concavity proximal to base of terminal process; terminal process short, rounded, bearing dense, short, stiff setae obscuring subterminal lobe and distal chitinous prominence; lateral surface of terminal process deeply concave; distolateral margin shallowly concave; distal chitinous prominence very short; genital pore subterminal. Second gonopod (Fig. 6C, D) very short; proximal lobe distinct, broadly rounded, with deep concavity on ventrolateral surface; terminal chitinous process unequally bilobed, larger ventral lobe rounded, with 4 terminal setae, small dorsal lobe crenulate.
Sternal surface ( Fig. 3A View FIGURE 3 ) sparsely punctate. First and second thoracic sternites separated by transverse row of granules and row of dense setae, first sternite triangular, coarsely granular on surface; second sternite broadly subrectangular, with granular margins. Third and fourth sternites fused, only faint indication of separation visible. Fifth, sixth sternites narrower than fourth sternite.
Abdomen triangular ( Fig. 3A View FIGURE 3 , 5E). First somite gently arched, with sharp transverse submedian ridge. Second somite very narrow. Third somite moderately broad, as wide as first somite; lateral margin divergent backwards, convex. Fourth somite broader and shorter than fifth, lateral margins slightly sinuous. Fifth somite with lateral margins nearly straight, slightly divergent backwards. Sixth somite longest, subrectangular, 1.45–1.72 times wider than long, lateral margin forming rounded shoulder at anterolateral angle. Telson roundly triangular, 1.13–2.22 times wider than long.
Description of adult females
Carapace similar to that of males, greatest width 1.16–1.18 of carapace length; distance between external orbital teeth 0.49–0.54 of cl.
Chelipeds generally similar to those of males, but chelae relatively smaller than those of males; chela length attaining 0.83 of cl, 1.91–1.77 longer than high.
Merus of third ambulatory leg 0.66–0.71 of carapace length, 3.02–3.26 times longer than wide; propodus of fifth leg 2.17–2.42 times longer than wide.
Abdomen ( Fig. 3B View FIGURE 3 ) broadly rounded, large, covering most of sternum when mature. First three somites ridged, second shorter than third. Fourth and fifth somites similar in shape, but former shorter than latter. Sixth somite broader than fifth, proximal margin slightly convex medially, anterolateral margin gently convex, anterior (distal) margin weakly concave. Telson transversely subtriangular. Anterior border of abdominal cavity densely covered with soft setae. Vulva (Fig. 6E, F) on sixth thoracic sternite subcircular in outline, with conspicuous subconical operculum directed mesially, basally demarcated by broad chitinous suture.
Coloration in life
Overall dorsal surface dark brown in life ( Fig. 1B View FIGURE 1 , 9A View FIGURE 9 ), occasionally with tinge of dark purple. Thoracic sternum and male abdomen whitish, female abdomen always brown ( Fig. 3B View FIGURE 3 ). other marginal granules, is discernible on either or both sides. The setation of the chelipeds and ambulatory legs is highly variable. Setation is better developed in fully matured males. It is generally well known that the shape of the abdomen changes with growth in brachyuran crabs, particularly dramatically so in females. The smallest female with a fully developed abdomen, completely covering the thoracic sternum, is CL 57.0 mm (Yamamoto, unpublished data).
(mainly from late March to April) (Yamamoto, unpublished data). Eggs are very small, measuring approximately 400µm in diameter. Zoea and megalopa larvae spend their planktonic stages in the sea ( Kobayashi, 2005). Adult crabs die in the sea after reproduction (Yamamoto, unpublished data).
Etymology The specific name refers to the islands where the new species inhabits.
Discussion
The taxonomy of Eriocheir has been a subject of debates in the last decade, and its current status is rather confusing (see Chu et al. 2003; Tang et al. 2003; Chan et al. 2005). It is convenient to summarize the recent arguments on the taxonomy or systematics of the genus in order to show our status how the treatment the taxa once referred to Eriocheir .
The first point of the disagreements is addressed in the ranking of the three named taxa, E. japonica (De Haan, 1835) , E. sinensis H. Milne Edwards, 1854 , and E. hepuensis Dai, 1991 . The former two species had been long considered to be specifically distinct (see Sakai 1976). Eriocheir hepuensis was first described by Dai (1991) as a subspecies of E. japonica . Based on morphometric and allozyme analyses, Li et al. (1993) concluded that these three taxa belong to a single species. On the other hand, based on larval morphology, Montú et al. (1996) discussed that E. sinensis is distinct from E. japonica . Guo et al. (1997) also recognized the three taxa as distinct species on the basis of extensive comparison of adult morphology. Gao and Watanabe (1998) showed that the genetic distance between Japanese and Chinese populations of mitten crab is much greater than the average distance among Japanese populations, also suggesting that E. japonica and E. sinensis are distinct. Using RAPD analysis, Lu et al. (2000) showed low genetic differentiation between the three taxa, possibly due to hybridization and artificial transplantation in aquaculture. Based on molecular phylogenetic analysis of the complete sequences of the nuclear DNA internal transcribed spacer (ITS) and portions of the mitochondrial genome corresponding to the cytochrome oxydase I (COI), Tang et al. (2003) supported low genetic differentiation between the three taxa and claimed that the three taxa could be ranked at most as subspecies of a single species, E. japonica . Chu et al. (2003) also showed that the three taxa are genetically very similar based on DNA sequence analysis of mitochondrial 16S rRNA, COI and the first internal transcribed spacer of nuclear rRNA, but they noted that there are a few constant differences distinguishing the three taxa.
The second point is the nomenclatural confusion regarding to E. recta Stimpson, 1858 , caused following Tang et al. (2004). Chan et al. (1995) showed that a species occurring in the eastern part of Taiwan, first identified with as E. recta by Sakai (1939), does not represent the true E. recta . They described E. formosa for the population in the eastern part of Taiwan, and designated a neotype for E. recta , a specimen of E. japonica collected nearby Zhuziang, China, close to near Macau, the original type locality of E. recta . This action effectively made E. recta as a junior synonym of E. japonica . Tang et al. (2003) claimed that they discovered a specimen of Eriocheir species from Zhujiang, China, which matched the original descriptions of E. recta by Stimpson (1858) and E. formosa by Chan et al. (1995), with molecular evidence confirming their conspecificity. Tang et al. (2003, 2004) argued that the name E. recta should be revived and that E. formosa should be synonymized with it. Finally Tang et al. (2004) formally designated their Zhuziang specimen, as the neotype of E. recta . Chan et al. (2005) published a rebuttal to the action of Tang et al. (2004), in which they reiterated that the action by Chan et al. (1995) is fully justified under current nomenclatural rules (see Chan et al. 2005 for details).
The third point is the generic assignment of the two described species, E. leptognathus Rathbun, 1913 and E. formosa , which is also a subject to disagreement. Sakai (1983) established a new genus Neoeriocheir to accommodate E. leptognathus based mainly on the difference in the structure of the third maxilliped. Subsequent authors ( Dai et al. 1986; Dai & Yang 1991; Chan et al. 1995) did not consider Neoeriocheir as a valid genus. Ng et al. (1999) revived Neoeriocheir for E. leptognathus , and further established a new genus Platyeriocheir for E. formosa . They provided evidence derived from both larval and adult morphology. The generic classification by Ng et al. (1999) has not been accepted by Tang et al. (2003) and Chu et al. (2003), as E. leptognathus , E. formosa and the other three taxa form a clade and as the genetic differentiation for the genes used at least among the two species, E. leptognathus and E. formosa , and the other three taxa is too low to justify separation at the genus level.
Our own morphological comparison, although the sample size is limited, would seem to support Guo et al.’s (1997) conclusion that E. japonica , E. sinensis and E. hepuensis are distinct, as some of the differentiating characters proposed by them, including the physiognomy of the carapace, the development of the fourth anterolateral tooth on the carapace, and the stoutness/slenderness of the ambulatory legs agree with the separation of morphotypes corresponding to the three taxa. We therefore follow Guo et al. (1997) in treating each of the three taxa as separate species. We fully concur with Chan et al. (2005) regarding to the nomenclature of E. recta and E. formosa . The action by Chan et al. (1995) is undoubtedly valid without doubt, satisfying all the provisions of the current code (ICZN, 1999). As Chan et al. (2005) noted, Stimpson (1858, 1907) clearly mentioned in his descriptions of E. recta that the indistinctly fourlobed front and the presence of a rudimentary fourth anterolateral tooth on the carapace. In E. formosa , the front is truncate and straight, and the fourth anterolateral tooth is completely absent. There is no doubt that E. recta and E. formosa are distinct. Tang et al.’s (2004) hypothesis is therefore untenable.
Although Ng et al. (1999) argued that Eriocheir s. l. was heterogeneous, recent genetic studies support the monophyly of the assemblage including E. leptognathus , E. formosa , and the three closely related taxa E. japonica , E. sinensis , and E. hepuensis ( Tang et al., 2003; Chu et al. 2003). However, although these species are in one group, the structure of the relationship shown by Tang et al. (2003) and Chu et al. (2003) is that E. leptognathus belongs to one clade, with E. formosa , E. japonica , E. sinensis and E. hepuensis in another clade. For the second clade, E. formosa belongs to a separate branch from E. japonica , E. sinensis and E. hepuensis . This phylogenetic pattern actually does not contradict the generic classification proposed by Ng et al. (1999), i.e. that three genera can be recognized. We therefore follow Ng et al.’s (1999) classification in this study.
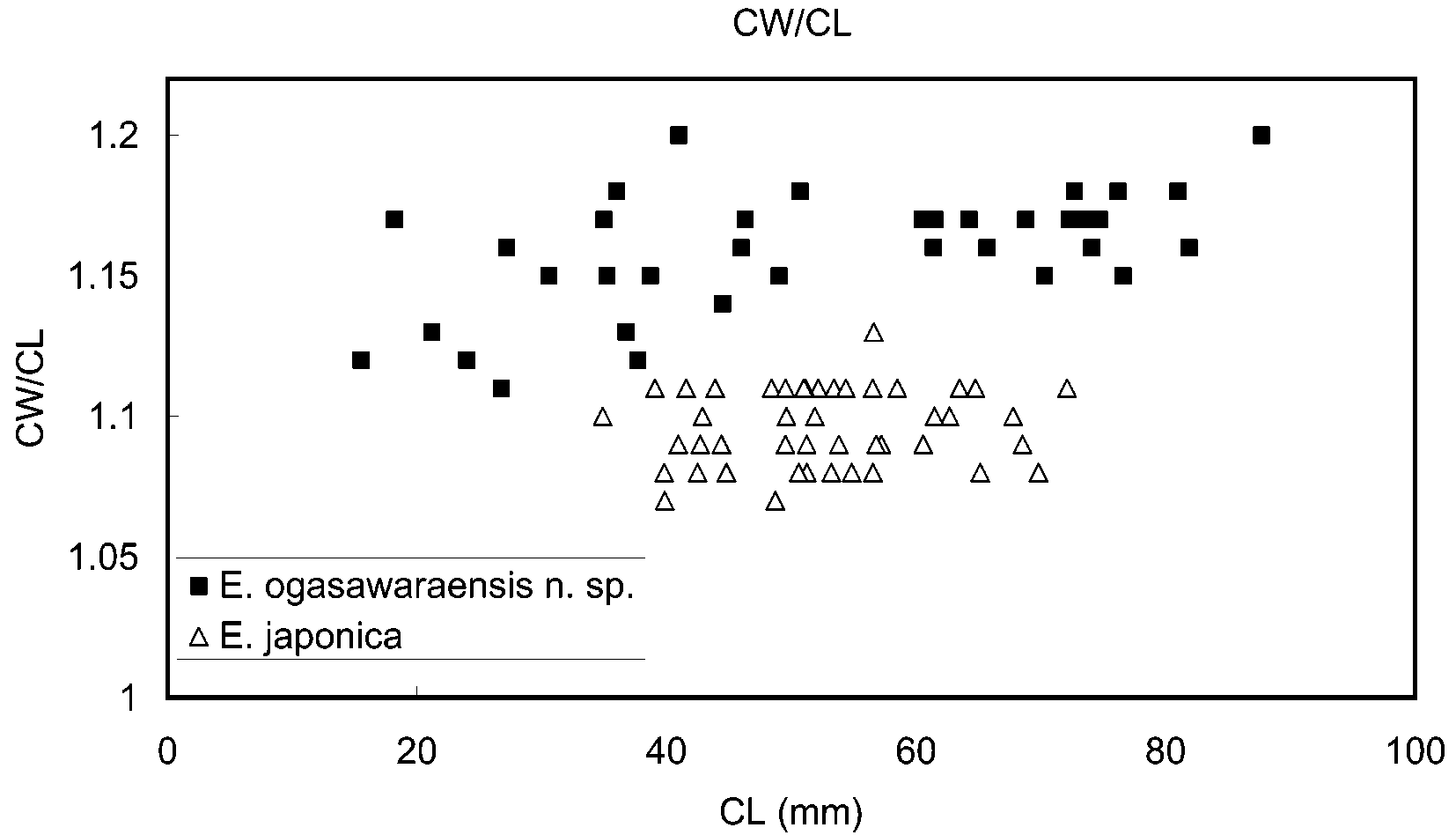
The present new species can clearly be assigned to Eriocheir on account of the structures of the front, third maxilliped, fingers of the cheliped and thoracic sternum, as well as the setation of the male chelipeds ( Ng et al., 1999). Among the three known species of Eriocheir s. s., E. japonica is most closely similar to E. ogasawaraensis . Kobayashi (2005) remarked that the Ogasawara population differed morphologically from the Japanese mainland population of E. japonica in (1) a relatively broader carapace, (2) absence of the fourth anterolateral tooth on the carapace, (3) relatively large size of reproductive individuals, and (4) relatively larger chela in both adult males and adult females, of which the ventral surface of the male chela is always lacks setae. The present study confirms that the carapace is broader in E. ogasawaraensis than in E. japonica at least in adults ( Fig. 7 View FIGURE 7 ). The ratio “width/length” of the carapace in adults (CL> 45.0 mm) is 1.15–1.20 in E. ogasawaraensis , 1.07–1.13 in E. japonica . Intraspecific variability in both E. ogasawarensis and E. japonica reduces the reliability of the character of the fourth anterolateral tooth. Nevertheless, this character is still useful in discriminating the two species. In E. japonica , the fourth anterolateral tooth is sometimes greatly reduced to a granule, being only slightly larger than other granules bordering the anterolateral margin, but it is never completely absent as in most specimens of E. ogasawaraensis . The setation of the male cheliped also allows differentiation between E. ogasawaraensis and E. japonica , although this character is subject to considerable variation in E. japonica , and thus not reliable. The ventral surface of the chela is always naked in E. ogasawaraensis , while it is occasionally covered by a dense mat of setae in E. japonica . The mats on the outer and inner surface are connected, most frequently in large specimens (> CL 60 mm). The ventral surface of small males of E. japonica (<CL 50 mm) is usually naked as in E. ogasawaraensis . Among the male specimens of E. japonica examined in this study, the smallest specimen having a covering of setae on the ventral surface of the chela is CL 49.0 mm (CBMZC 8570).
Furthermore, E. ogasawaraensis differs from E. japonica in the following other characters. The dorsal surface of the carapace is nearly transversely flat in E. ogasawaraensis ( Fig. 2B View FIGURE 2 ), whereas it is slightly convex in E. japonica . The slightly elevated gastric region makes the dorsal surface of the carapace appear convex in E. japonica . The third anterolateral tooth is smaller than the second anterolateral tooth in E. ogasawaraensis (Fig. 5B), rather than being subequal in E. japonica (Fig. 8A). The ventral margin of the epistome is usually nearly flat or faintly sinuous with a trace of a median lobe in E. ogasawaraensis (Fig. 5C), whereas it is similarly sinuous but and provided with a distinct median tubercle in E. japonica (Fig. 8B). The lateral margins of the sixth abdominal somite of males are more noticeably arched in E. ogasawaraensis (Fig. 5E) than in E. japonica (Fig. 8C). The first gonopod is less stout in E. ogasawaraensis than in E. japonica (8.1–8.5 times longer than broad in E. ogasawaraensis in contrast to 6.5–7.0 in E. japonica ; cf. Fig. 6A and Fig. 8D). The detailed texture of the coloration is also different between the two species. The dorsal surface of the carapace is almost uniformly dark brown in E. ogasawaraensis ( Fig. 9A View FIGURE 9 ), whereas a complicated reticulate pattern of black and pale yellowish gray is found on the carapace in E. japonica ( Fig. 9B View FIGURE 9 ). The female abdomen is always brick brown in E. ogasawaraensis ( Fig. 3B View FIGURE 3 ), rather than normally white in E. japonica .
Eriocheir sinensis and E. hepuensis differ from E. ogasawaraensis in the clearly delineated fourth anterolateral tooth on their carapaces and the proportionally narrower carapaces (width/length ratio of the carapace 1.08–1.12), with a more convex dorsal surfaces in the former two species. The median notch of the front is deep in the former two species, but very shallow in E. ogasawaraensis . The posterior margin of the epistome is provided with a small, blunt median tooth or tubercle in E. hepuensis or with a prominent median tooth in E. sinensis .
Miyake (1970) was the first carcinologist to E. japonica from the Ogasawara Islands, although he only listed the species without any comments. In their list of the brachyuran crabs known at that time from the Ogasawara Islands, Takeda & Miyake (1976) also included E. japonica . Muraoka (1998) listed 16 specimens from Chichijima Island under E. japonica . So far, from the Ogasawara Islands, E. japonica s. s. has not yet been collected, and therefore, these early records are all referred to E. ogasawaraensis .
Available data strongly suggests that the present new species is endemic to the Ogasawara Islands, although details of its distribution remain incomplete. The crab species is so far recorded from four main islands, Chichijima, Hahajima, Anijima and Otoutojima islands. Conservation status of the new species is urgently needed to be assessed.
| NTOU |
Institute of Marine Biology, National Taiwan Ocean University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
Genus |
Eriocheir ogasawaraensis Komai
| Komai, T., Yamasaki, I., Kobayashi, S., Yamamoto, T. & Watanabe, S. 2006 |
Eriocheir japonicus
| Kobayashi, S. 2005: 17 |
| Yamamoto, T. 2004: 16 |
| Muraoka, K. 1998: 52 |
| Miyake, S. 1983: 174 |
| Takeda, M. & Miyake, S. 1976: 112 |
| Miyake, S. 1970: 289 |