Fulica Linnaeus, 1758
|
publication ID |
https://doi.org/10.11646/zootaxa.4626.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:6CC12BAF-968F-4BDE-9315-340AF12A76EC |
|
persistent identifier |
https://treatment.plazi.org/id/0E538795-355D-FFE1-FF1F-FBDAFADBFD4D |
|
treatment provided by |
Plazi |
|
scientific name |
Fulica Linnaeus, 1758 |
| status |
|
Genus Fulica Linnaeus, 1758 View in CoL
†Mascarene Coot Fulica newtonii Milne-Edwards, 1867b
Poules d’eau ( waterhen) Pretorius, [1666] (in Hume & Winters, 2016, p.5) ( Mauritius); Granaet, [1666] (in Barnwell, 1948, p.40); François Martin in [ 1665 and 1667] (in Lougnon 1992, pp. 40, 43) ( Réunion); Carpeau du Saussay, [1666] (in Lougnon, 1992, p.51) ( Réunion); Le Breton, [1671] (in Lougnon, 1992, p.135) ( Réunion); Dubois, 1674, p.169 ( Réunion); Leguat, 1708 (French edition), p.71 ( Mauritius); Oliver, 1898, p.77
Moor-hen Leguat, [in 1693] 1708 (English edition), p.71
Fulica newtonii: Milne-Edwards, 1867b , pp.203, 204(table), 211(table), 215, 216, 217, pl.10, 1866–1873 [1874], p.55; Oliver, 1891, p.369; Pitot, 1914, p.89; Olson, 1977, p.363; Cowles, 1987, p.96, 1994, p.92; Probst, 1997, p.48; Taylor & van Perlo, 1998 , p.61; Probst & Brial, 2002, p.53; Mourer-Chauviré et al. 2006, p.43; Garcia et al. 2014, p.105; del Hoyo & Collar, 2014, pp.342,773; Hume, 2017, p.140
Fulica newtoni: Milne-Edwards, 1869 –1871, p.139, fig.1–2, 1896a, p.76, 1896b, p.118; E. Newton, 1888, p.552; E. Newton & Gadow, 1893, pp.282, 292–293, figs,1–11, 1895, p.233; Forbes, 1893a, p.544; Sharpe, 1894, p.209; Emerez de Charmoy, 1903, p.7; A. Newton, 1905, p.142; Carié, 1916, p.109; Sclater, 1924, p.109; Lambrecht, 1933, pp.470, 481; Guérin, 1942, 2(12), 1950, p.405; Berlioz, 1946, p.7; Greenway, 1958, p.119, 1967, p.119; Luther, 1970, p.187, 1986, p.183, 1986 [2005], p.183; Barré & Barau, 1982, p.32; Cheke, 1987, p.35, 2013b, p.13; Balouet, 1990, p.45; Livezey, 2003, p.2; Adams et al. 2003, p.344; Hume et al. 2006, p.17; Cheke & Hume, 2008, p.135, tbls.3.1,4.1, pls.3,6; Hume & Walters 2012, p.120; Hume, 2013, p.219, 2014a, p.38; Safford & Hawkins, 2013, pp.45,348 (lapsus)
Palaeolimnas newtoni: Forbes, 1893a , p.544; Rothschild, 1907a, p.150, 1907b, p.198; Brodkorb, 1967, p.130
Fulica ( Palaeolimnas?) newtoni: Oustalet, 1897, p.99 View in CoL
Paludiphilus newtoni: Hachisuka, 1953, p.154
Holotype: None designated.
Syntypes: Milne-Edwards did not specify holotype material when describing Fulica newtonii , but three presumably unassociated elements, pelvis , tibiotarsus, tarsometatarsus, were figured ( Milne-Edwards 1867b: pl.10). He returned these specimens to UMZC, and by using details from the labels and by matching them to the illustrations, I have been able to definitely assign the following as Milne-Edwards’ syntypes: Pelvis , UMZC 573 (pl.10, 1–4); Tibiotarsus, UMZC 573 (L) (pl.10, 11–15). There are two other tibiotarsi, right and left, with the same cata- logue number ( UMZC 573), but these are the largest known (see Appendix 2, Table 15) and may be associated. They are too large to belong to the illustrated syntype series. Furthermore, the tarsometatarsus (pl.10, 5–10) in Milne-Edward’s plate is from the left side, whereas a tarsometatarsus ( UMZC 573) listed in the UMZC catalogue as the possible holotype is from the right side. Another possibility is that the tarsometatarsus is figured in reverse, in which the original plate is reproduced in a mirror image, i.e. the bone from the right side will appear as a left. This was a standard procedure in lithography ( Weaver 1964), but unfortunately there are no diagnostic erosional features on the UMZC 573 bone to link it with the illustration. There are also four other left tarsometatarsi in the UMZC collections all catalogued UMZC 298.aa, so the syntype tarsometatarsus cannot be confidently identified and is not considered further.
Measurements: See Appendix 2.
Type locality: Mauritius, Mascarene Islands.
Distribution: Mauritius and Réunion, Mascarene Islands.
Etymology: After Sir Edward Newton ( 1832–1897), British assistant colonial secretary on Mauritius, and a keen naturalist who organised the collection of many subfossil specimens on Mauritius and Rodrigues.
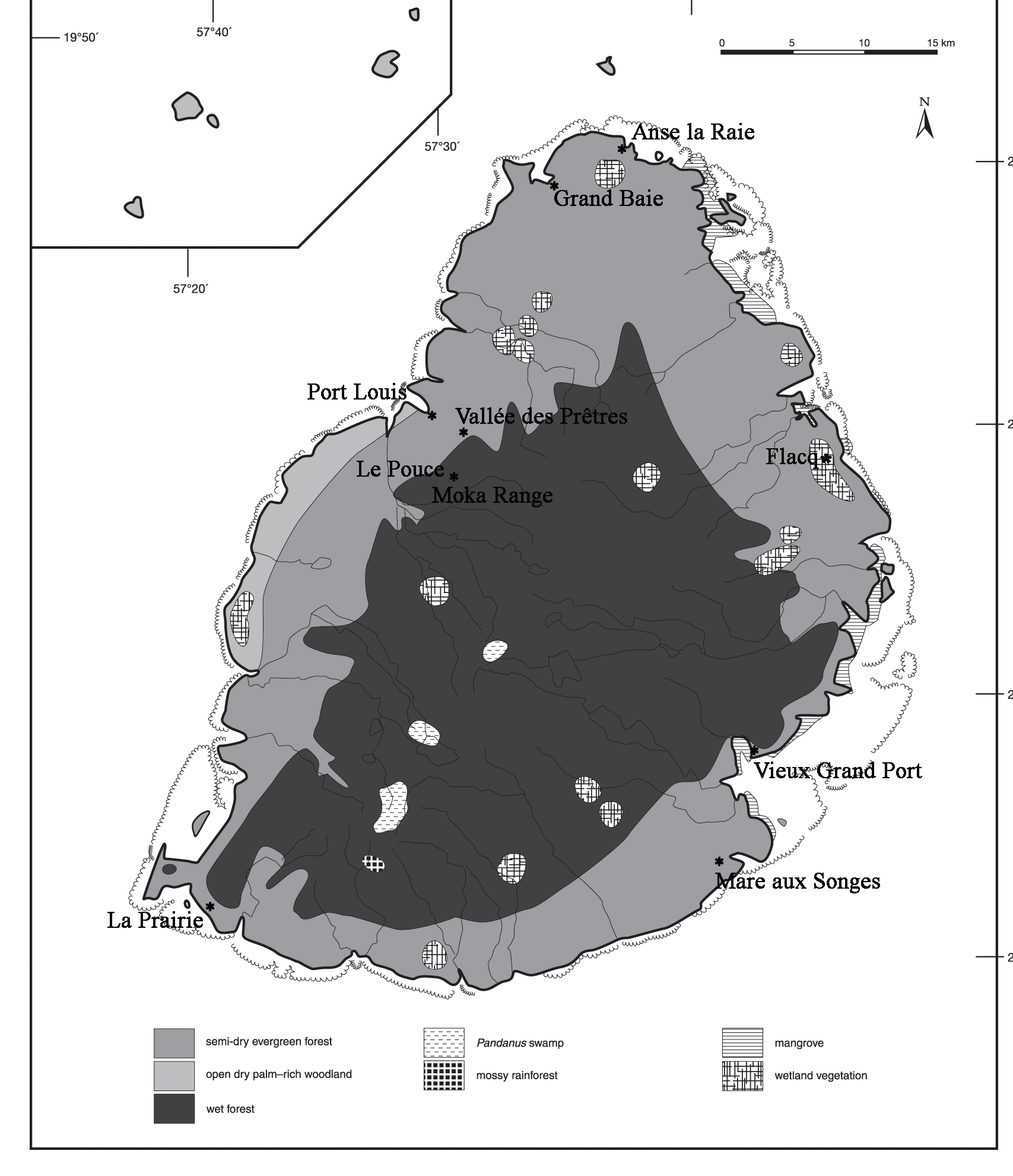
Referred fossil material: Rostrum, UMZC 298.aa; Vertebrae (cervical), NHMUK u/r; NHMUK u/r; NHMUK u/r; Scapula, MNHN MAD 8856 (Lp); MNHN MAD 8856 (L); Sternum, UMZC 298.aa; Humerus , MNHN MAD 6571 (R); MNHN MAD 6570 (R); NHMUK u/r (Lp); NMHUK u/r (Lp); NHMUK u/r (Lp); NHMUK u/r (Rd); UMZC 298 (L); UMZC 298 (R); UMZC 298 (L); Radius, DRP 237.B4 (R); MNHN MAD 8856 (R); Ulna , UMZC 298 (R); UMZC 298 (L) juv.; Carpometacarpus, DRP 20.137 (R); Pelvis , MNHN MAD 6505; NHMUK u/r; NHMUK u/r; UMZC 298.aa; UMZC 298.aa; DRP 324.B1; Femur, UMZC 298.aa (L); LWM u/r (R); LWM u/r (R); LWM u/r (R); LWM u/r (L); LWM u/r (Ld); Tibiotarsus, BMB 10179 (L); BMB 101748 (L); DRP294.B1 (Rd); MNHN MAD 6504 (R); MNHN MAD 6503 (L); MNHN MAD 6562 (L); MNHN MAD 6823 (Rd); MNHN MAD u/r (L); MNHN MAD u/r (L); NHMUK u/r (L); NHMUK u/r (Rd); NHMUK u/r (L) juv.; NHMUK u/r (L) juv.; NHMUK u/r (L); UMZC 1004 (R); UMZC 1004 (R); UMZC 1004 (L); UMZC 1004 (R); UMZC 1004 (R); UMZC 1004 (L); UMZC 1004 (L); UMZC 1004 (R); UMZC 1004 (R); UMZC 1004 (L); UMZC 573 (R); UMZC 573 (L) (largest known, possibly associated specimens); Tarsometatarsus, MNHN MAD 6501 (R); MNHN MAD 6508 (L); NHMUK A1353 (R); NHMUK A1353 (L); NHMUK u/r (L); NHMUK u/r (R); NHMUK u/r (L); NHMUK u/r (Ld); NHMUK u/r (Lp); NHMUK u/r (R); NHMUK u/r (R); NHMUK u/r (L); NHMUK u/r (R); NHMUK u/r (R); NHMUK u/r (R); NHMUK u/r (Lp); NHMUK u/r (R); NHMUK u/r (Rd); NHMUK u/r (R) juv.; UMZC 573 (R); Phalanx, MI u/r (Flacq). Additional Réunion material not examined by me (in Cowles 1994): Premaxillae LAC 1993.44; Pelvis LAC 1993.38; Sternum LAC 1993.39; Tibiotarsus LAC 1993.40 (L); LAC 1993.41 (R); Fibula LAC 1993.43; Phalanges X5 LAC 1993.45; Vertebrae X2 LAC 1993.46. A recent discovery of a phalanx at a marsh site in the Flacq area, north-east Mauritius ( Fig. 2 View FIGURE 2 ) in 2018 confirms its presence in the northern wetlands, but F. newtonii probably occurred in all suitable wetland habitat on Mauritius.
Revised diagnosis: Rostrum ( Fig. 32 View FIGURE 32 ): os premaxillare short and weakly pointed; in dorsal view, os nasale narrow, not covering narial openings; in lateral view, foramina neurovascularia, present, small and circular with limited extent; os nasale narrow; in ventral view, deep, distinct sulcus.
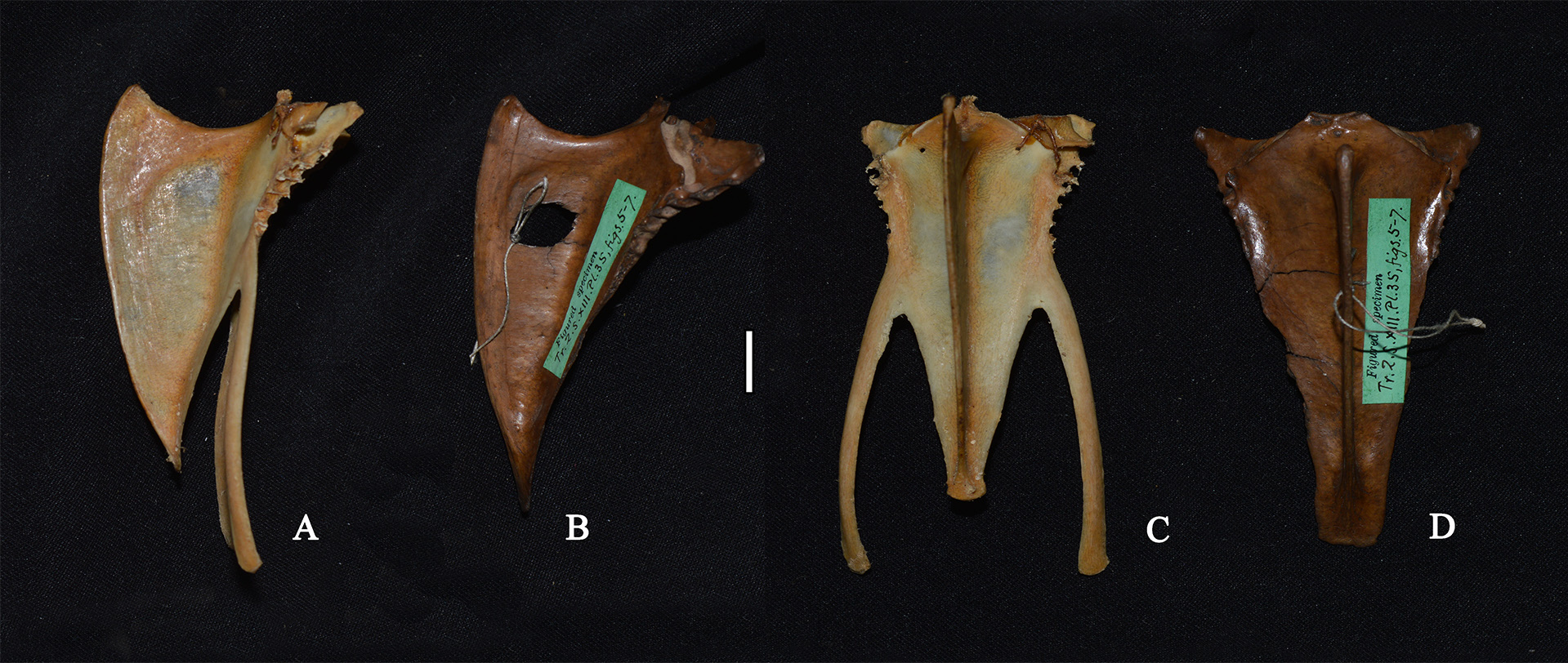
Scapula ( Fig. 33 View FIGURE 33 ): in lateral view, narrow; margo dorsalis distinct; facies articularis humeralis shallow; acromion blunt, rounded and directed dorsad; tuberculum coracoideum distinct.
Sternum ( Fig. 34 View FIGURE 34 ): long and extremely narrow caudally; in dorsal view, processus craniolateralis broad and project laterally; spina interna short, square-shaped, and slightly bifurcated; in lateral view, apex carinae extends cranially less than rostrum sterni.
Humerus ( Fig.35E View FIGURE 35 ): shaft strongly curved dorsoventrally; in caudal aspect, tuberculum ventrale pronounced, incisura capitis shallow; fossa pneumotricipitalis variable, either with or without pneumaticum; tuberculum dorsale oval shaped and reduced in size; sulcus humerotricipitalis shallow; in cranial view, sulcus ligamentosa transversus shallow; crista bicipitalis, short, weakly square-shaped; fossa m. brachialis shallow; distinct processus supracondylaris dorsalis.
Radius ( Fig. 35A View FIGURE 35 ): sulcus tendinosa deep extending proximad approximately ¼ of total length radius length; facies articularis radiocarpalis deflected ventrally; in ventral aspect, depressio ligamentosa deeply excavated.
Ulna ( Fig. 35C View FIGURE 35 ): short and strongly arched dorso-ventrally proximally; in caudal aspect, olecranon distinct; processus cotylaris dorsalis hooked with deep impressio scapulotricipitis; sulcus intercondylaris deep.
Pelvis ( Fig. 35G View FIGURE 35 ): extremely long and narrow; in dorsal view, sulcus antitrochantericus extend laterally beyond the ala preacetabularis; canalis iliosynsacralis opens cranially; few indistinct foramina intertransversariae; in lateral view, foramen acetabuli smaller in size than foramen ilioischiadicum; crista iliaca dorsalis arched; in ventral view, one pronounced processus costalis; ala ischii directed caudad.
Femur ( Fig. 35I View FIGURE 35 ): extremely robust; in cranial view, shaft curved medially and directed caudally at the proximal end; crista trochanteris strongly deflected mediad; linea intermuscularis indistinct; sulcus patellaris deeply exca- vated; epicondylus lateralis distinct; condylus medialis extends the same distance distally as condylus lateralis; in medial aspect, fovea ligamentosa capitis deep and circular; crista trochanteris extends caudad, offsetting centrally facies articularis acetabularis; shaft strongly arched dorsally; in caudal aspect, impressiones obturatoriae indistinct; linea intermuscularis caudalis distinct, forming a ridge; fossa poplitea shallow; condylus medialis smaller than con- dylus lateralis; trochlea fibularis deeply excavated.
Tibiotarsus ( Fig. 35K View FIGURE 35 ): long and robust; in cranial view, crista cnemialis forms a distinct proximal pointing ridge; sulcus extensorius deeply excavated, extending proximad; canalis extensorius oval-shaped; pons supratendin- eus wide and slightly arched, and situated mediad; crista cnemialis lateralis extends distad; in caudal aspect, crista cnemialis cranialis strongly hooked; shaft shallow; condylus lateralis small, weakly rounded; depressio epicondy- laris lateralis deep.
Tarsometatarsus ( Fig. 35M View FIGURE 35 ): long and robust; in dorsal aspect, trochleae metatarsorum weakly splayed; trochlea metatarsi II directed slightly plantad; impressiones retinaculi extensorii indistinct; two or more foramina vascularia proximalia; in plantar aspect, surface area of foramen vasculare distale deeply excavated forming a large circle; fossa metatarsi I deep and extends proximad; on proximal end, canal for tendon of musculus flexor digitorum longus; deeply incised sulcus for tendon of musculus flexor perforatus digiti II and shallow sulcus for tendon of musculus flexor hallucis longus present.
Description and comparison: For comparison with other Mascarene rallid genera, see under Aphanapteryx . Fulica newtonii differs from F. atra by the following characters:
Rostrum: Larger, otherwise similar.
Scapula: Proportionately shorter; proximal end more robust; tuberculum coracoideum more pronounced; margo dorsalis less defined; extremitas caudalis blunter.
Sternum: in dorsal view, processus craniolateralis more prominent, extending more laterad; rostrum sterni more square-shaped, not bifurcated; in lateral view, apex carinae extends less craniad; otherwise similar.
Humerus: Comparatively short; incisura capitis shallower; in cranial view, fossa m. brachialis extends further proximad; otherwise similar.
Radius: proportionately more robust, especially at proximal and distal ends; in caudal aspect, tuberculum aponeurosis more pronounced; in cranial aspect, caput radii more deeply incised; sulcus tendinous more deeply excavated.
Ulna : comparatively short in total length; processus cotylaris dorsalis shorter, otherwise similar.
Pelvis : much larger, otherwise similar.
Femur: much larger, approximately 19% difference in total length and more robust, especially on proximal and distal ends (see Appendix 2, Table 12 View TABLE 12 ); in cranial view, crista trochanteris larger and directed more distad; in caudal aspect, sulcus more deeply excavated; otherwise similar.
Tibiotarsus: proportions differ greatly from F. atra , being closer to Porphyrio , i.e. long-legged; much larger, approximately 32% difference in total length and more robust, especially on proximal and distal ends (see Appendix 2, Table 13 View TABLE 13 ); in cranial aspect, crista fibularis comparatively short; sulcus extensorius more deeply excavated.
Tarsometatarsus: proportions differ greatly from F. atra , being closer to Porphyrio , i.e. long-legged; much larger, approximately 24% difference in total length, and more robust, especially on distal end.
Remarks: Milne-Edwards (1867b) described F. newtonii from a series of bones found at the Mare aux Songes marsh, Mauritius. From my comparison with other similar-sized species, it measured around 35 cm in total length and may have had a body mass of up to 1.2 kg. There is also a nearly complete growth series for juvenile tibiotarsi ( Fig. 36 View FIGURE 36 ). Despite having a reduced sternum (see Appendix 2), Newton and Gadow (1893) noted that there was only slight reduction in the carina sterni ( Fig. 34 View FIGURE 34 ), so it was clearly able to fly. F. newtonii has also been identified from subfossil material discovered on Réunion and, because the two island populations are undifferentiated, Mourer- Chauviré et al. (1999) suggested that it still had the capability to fly between Mauritius and Réunion, possibly until comparatively recent times.
The extremely large pelvic and leg elements of F. newtonii , compared with the reduced pectoral and wing elements, are similar proportionately to the world’s largest coots, Chatham Island Coot F. chathamensis Forbes, 1892b and New Zealand Coot F. prisca Hamilton, 1893 . These coots had long necks, disproportionately long legs and toes, and an upright stance ( Worthy & Holdaway 2002; Tennyson & Martinson 2006), very similar to Porphyrio gallinules. This is in complete contrast to all other extant Fulica , which are swimming birds with shorter legs, and is most likely a result of the New Zealand birds being more terrestrial (Worthy & Holloway 2002). The Mascarene Coot had a similar body shape with long neck and legs, which suggests that it was also probably more terrestrial. The relationships of F. newtonii are unclear, but it is thought to be a derivative of the Eurasian Coot Fulica atra Linnaeus, 1758 ( Olson 1977) or Red-knobbed Coot F. cristata Gmelin, 1789 , of Madagascar ( Mourer-Chauviré et al. 1999). However, due to the sister relationships of some other Madagascar avian taxa, e.g. Madagascar Teal Anas bernieri ( Hartlaub 1860) and Grey Teal Anas gracilis Buller, 1869 of Australasia ( Mitchell et al. 2014a), and Elephant birds Aepyornis sp. and New Zealand kiwi Apteryx sp. ( Mitchell et al. 2014b), an African or Malagasy origin may not be the case.
Ecology: Virtually nothing was recorded about the ecology of F. newtonii , other than it was universally called Poule d’eau (waterhen) and considered variably good to eat. François Martin (in Lougnon 1992: 40) made the first mention in 1665 at Saint-Gilles on Réunion, recording the abundance of coots and other birds, and the ease at which they could be caught (my translation):
Various kinds of birds approached us, but so familiar that some even came to rest on our shoulders. The river basin was covered in geese and waterhens, and the depths full of fish. We had plenty of everything and of our choice, since a small baton was enough to kill parrots and other birds; that one could catch the pigs and goats by running [after them], and the geese and the waterhens allowed one to approach [so closely] as to almost take them by hand. We sent them all on board [ship].
But when Martin returned from Fort-Dauphin, Madagascar, two years later in 1667, he noted the destructive carnage caused by the inhabitants (in Lougnon 1992: 43; my translation):
When some of the vessels of the fleet [of marquis de Mondevergue] were forced to sail from the Mascarene island by the hurricane that surprised them there in February [1667], there remained many people left onshore, whom were to be picked up afterwards. During the time that these people stayed on the island, they created unbelievable commotion among the flocks, game and gardens. We saw neither geese nor waterhens on the Étang de St. Paul which was formerly covered in them, and one was obliged to go to three or four miles from the dwelling to find there goats and pigs.
Carpeau du Saussay in 1666 (in Lougnon 2002: 51) further commented on the abundance of birds, and stated (my translation):
The birds are abundant and very familiar. Above all, we saw an infinity of turtle doves, pigeons, parrots, waterhens, geese and ducks; only sticks and stones were needed to kill them.
The account from the log of Le Breton in 1671 (in Lougnon 1992: 135) noted that the Mascarene coot was still abundant (my translation):
There are plenty of fish, especially mullet and eels, as well as all kinds of river birds. This is admirable to see, also teals [ Anas theodori Newton & Gadow, 1893 ] and wild geese [ Réunion Sheldgoose Alopochen kervazoi ( Cowles, 1994)] which are smaller than those of Europe. There are also good numbers of waterhens, but they are not good to eat.
Sieur Dubois (1674: 169), who was on Réunion from March 1671 – September 4, 1672, not only gave the only colour description, but also the last account of the coot on Réunion (my translation):
Waterhens, which are as large as fowls. They are always black, and have a large white crest on the head.
The ‘large white crest’ refers to the bare frontal shield that occurs in coot and gallinule genera, including Fulica ( Ripley 1977; Taylor & van Perlo 1998 ).
The coot was first mentioned on Mauritius by a VOC (Vereenigde Oost-Indische Compagnie [Dutch East India Company]) book-keeper, Jacob Granaet, who arrived there on July 30, 1666 ( Barnwell 1948; Hume & Winters 2016), and noted (from Barnwell 1948: 42):
Within the forests dwell parrots, turtle and other wild doves, mischievous and unusually large ravens [ Lophopsittacus mauritianus ], falcons [ Mauritius Kestrel Falco punctatus Temminck, 1821 ], bats and other birds whose names I do not know, never having seen such before….Water-fowls such as geese [ Mauritius Sheldgoose Alopochen mauritiana Newton & Gadow, 1893 ], teals, waterhens and flamingos are found among the marshes, very numerous, especially the teals which are so tame they can be killed with sticks; all are fat and pleasant to eat.
They were briefly mentioned again in 1666 by Pretorius, who had stayed on Mauritius for three years (in Hume & Winters 2016: 5), and wrote:
…the teals [ Anas theodori ] and waterhens are always in the water, and all live together in peace and quiet.
In reference to Pretorius’s remark about the Mascarene coots’ peaceful existence, even though flight must have been impaired to some degree, one of a pair of associated humeri is pathological and exhibits a serious, but healed fracture on the distal end ( Fig. 37 View FIGURE 37 ). I have also seen similar healed wing injuries in subfossil remains of Echo Parakeet Psittacula echo ( Newton & Newton, 1876) and Pink Pigeon Nesoenas mayeri (Prévost & Knip, 1843) . This suggests that adult birds could recover from injuries that would likely have otherwise ended in premature death if predatory terrestrial mammals were present.
The Mascarene Coot probably had similar habits to F. atra (see Taylor & van Perlo 1998 ), nesting in aquatic vegetation alongside water, and feeding on vegetable matter, aquatic seeds and invertebrates.
Extinction: Despite their sometimes inadequate culinary qualities, both the Mauritius and, especially Réunion populations of Mascarene Coot were heavily hunted. Martin, in 1665, recorded an abundance of coots on the Étang St. Paul on the northwest coast, the largest lake and one of the most densely human-populated areas on Réunion dur- ing the 17th century ( Lougnon 1992; Blanchard 2000), but when he returned two years later they had been hunted out. They were still recorded as numerous elsewhere until the early 1670s, but not mentioned again thereafter. Black rats were accidentally introduced after 1672, and reached plague proportions by 1676 ( Cheke & Hume 2008; Hume 2013), so it is not known what effect, if any, they had on these birds, but the Mascarene Coot survived alongside rats on Mauritius for centuries ( Hume 2013). As cats were introduced in the 1680s ( Cheke & Hume 2008), and that F. newtonii was confined to lowland wetlands, it is likely that over-hunting and later cat predation exterminated the species on Réunion at some point after 1672, as Feuilley (1705), who gave a detailed account about the avifauna, including the native waterbirds, failed to mention the coot during his visit in 1704. In 1710 and just 40 years after the first settlement, Antoine Boucher, the French East India Company’s store keeper and treasurer, condemned the company’s management of Réunion and the wanton slaughter of the fauna ( Cheke & Hume 2008; Hume 2014b). He considered the settlers’ insatiable greed solely accountable for the faunal disappearance, and noted at the time of writing that almost all of the larger land birds, including F. newtonii were either extinct or nearly so.
The Mascarene Coot was last mentioned in 1693 on Mauritius when it was considered rare ( Leguat 1708), so presumably it succumbed to feral cat predation shortly thereafter, as did the other Mauritian endemic rails.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Fulica Linnaeus, 1758
| Hume, Julian Pender 2019 |
van
| Perlo 1998: 61 |
newtoni :
| Hachisuka 1953: 154 |
Palaeolimnas newtoni :
| Forbes 1893 |
Fulica newtoni :
| Milne-Edwards 1869 |
Fulica newtonii
| : Milne-Edwards 1867 |
Fulica ( Palaeolimnas ?) newtoni : Oustalet, 1897 , p.99
| Linnaeus 1758 |