Fergusobia rosettae Davies
|
publication ID |
https://doi.org/10.11646/zootaxa.3889.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:BBBE41C7-C8F1-476D-B136-80D11743F43D |
|
DOI |
https://doi.org/10.5281/zenodo.5612486 |
|
persistent identifier |
https://treatment.plazi.org/id/1136879A-8762-943B-FF77-B012BFB3F85B |
|
treatment provided by |
Plazi |
|
scientific name |
Fergusobia rosettae Davies |
| status |
|
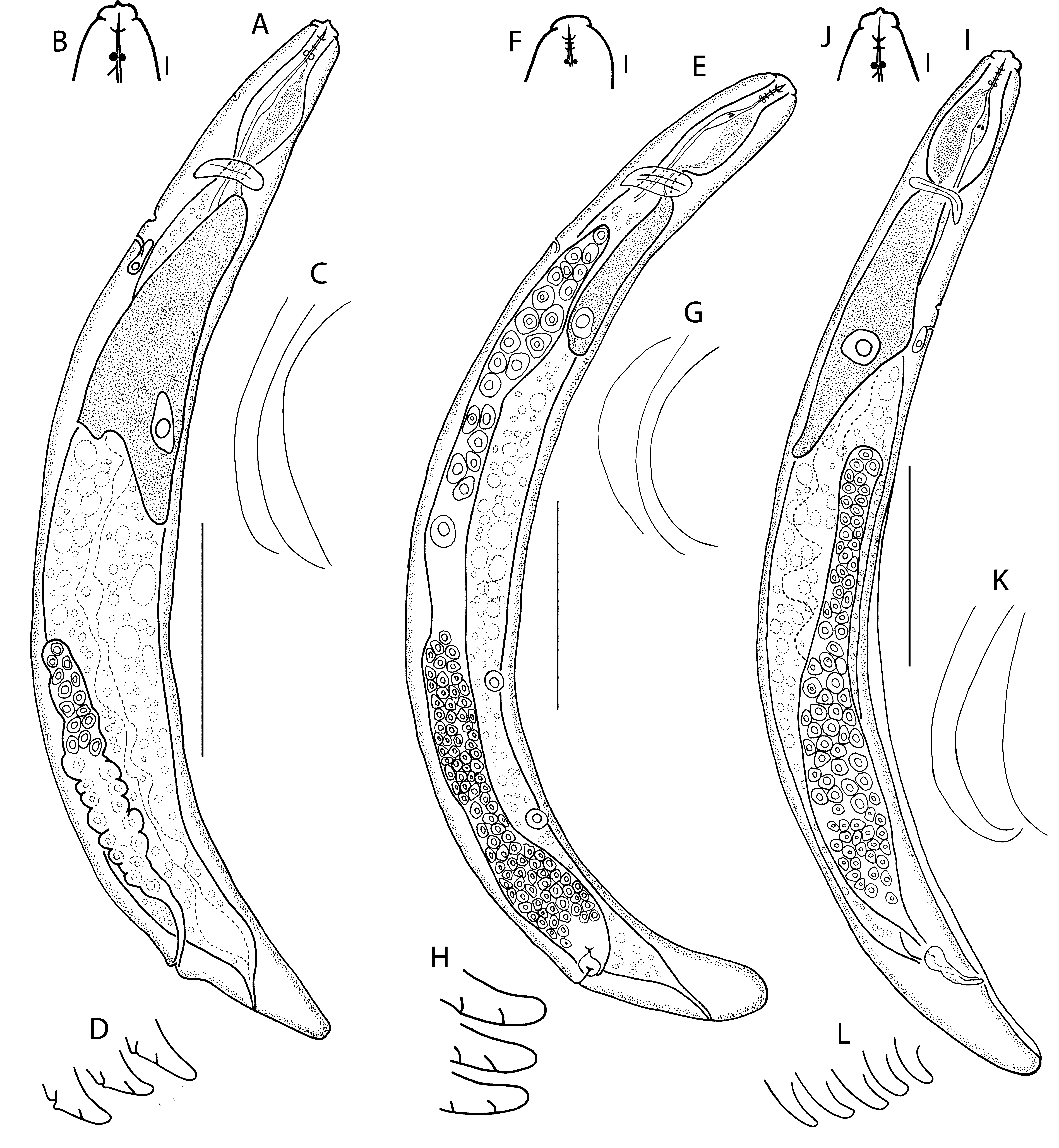
Description of Fergusobia rosettae Davies n. sp.
( Figs 1 View FIGURE 1 , 3 View FIGURE 3 A, 4A)
= Fergusobia MSp 45 apud Davies et al., 2012a
Measurements. Table 2 View TABLE 2 .
Material examined. Holotype, parthenogenetic ♀, roadside at Yellow Waterhole, ~ 2 km north of Kennedy in coastal eastern QLD ( 18º11.30’S, 145º56.66’E). From basal shoot bud (‘rosette’) galls on Melaleuca quinquenervia (Cav.) S.T. Blake 1958 , collected by K.A. Davies, 10.viii.2006 and 18.viii.2007. On slide with a paratype ♂, deposited at the ANIC, Canberra, ACT, Australia.
Paratypes, WINC, The University of Adelaide, SA, Australia, 3 parthenogenetic ♀s, 1 pre-parasitic infective ♀, 10 ♂ s, slide numbers WNC 2480; Queensland Museum, Brisbane, QLD, Australia, 10 parthenogenetic ♀s, 2 pre-parasitic infective ♀s, 12 ♂ s; and at the USDA Nematode Collection, Beltsville, MD, USA 1 parthenogenetic ♀ and 1 ♂. 15 parthenogenetic ♀♀, 4 pre-parasitic infective ♀♀, and 24 ♂♂, collected from the above location on 10.viii. 2006 and 18.viii. 2007.
Description. Parthenogenetic female. Body shape arcuate when heat relaxed; relatively small (< 0.3mm long); relatively broad compared to length; larger than amphimictic pre-parasitic females and smaller than males; body narrows behind vulva to form a short conoid tail. With light microscope, cuticle appears smooth, and longitudinal striae not apparent in sub-cuticle. Lateral fields not seen.
Cephalic region 5–7 µm wide, ~ 80% diameter of body at anterior end, off-set, 1 – 2 µm high, unstriated; lateral view with rounded outline and circum-oral area distinctly raised ~ 1 µm in most specimens. Stylet with cone ~ 40% of length; basal knobs just higher than wide, ~ 2 µm wide at base, rounded.
Orifice of dorsal pharyngeal gland ~ 1 µm posterior to stylet knobs. Anterior fusiform part of digestive tract occupying 53–74% of body diameter, length 3 times diameter of tract. Lumen of tract broadens at distal end of dorsal pharyngeal gland. Pharyngeal glands extending over intestine, large, diameter ~47–75% of body diameter, distance from head to end of glands being 45–66% (mean 55%) of total body length. Gland nucleus prominent, with obvious nucleolus.
Secretory/excretory pore with obscure duct, opens posterior to nucleus of pharyngeal gland; secretory/ excretory cell ovoid. Hemizonid 4 or 5 annules in front of secretory/excretory pore.
Reproductive tract variable in length, extending to secretory/excretory pore, part-way along pharyngeal gland, to base of gland or not reaching it; flexed in 1/ 17 specimens examined; oviduct usually with two oocytes per row; quadricolumella not smooth, uterus not seen containing eggs, apparently not extensile; vulva a simple transverse slit with protruding lips in some specimens; no vulval plate. Anus pore-like. Tail relatively short, conoid; length 1–1.5 times anal body diameter; bluntly rounded tip.
Infective pre-parasitic female. Infects mature larval stage of Fergusonina sp. or pupa. Arcuate to open-C shape when relaxed by heat; relatively slender; maximum body diameter at mid-body length; body tapers gradually behind vulva. Cuticle obscurely annulated, appears smooth; longitudinal striae not apparent with light microscope; lateral fields not seen.
Cephalic region barely offset, domed shape; circum-oral area rounded; stylet slender, weakly sclerotised with tiny basal knobs ~ 1.5 µm wide; cone ~ 40% of length.
Orifice of dorsal pharyngeal gland not seen. Anterior fusiform part of digestive tract little expanded, occupying 63–66% of body diameter, length 3.3–3.5 times diameter. Pharyngeal glands extending over intestine, diameter 54 (36–66)% of body diameter, extending over intestine, distance from head to end of glands being 34 (29–48)% of total body length.
Secretory/excretory pore opens behind pharyngeal glands or opposite gland nucleus; duct obscure; secretory/ excretory cell not seen. Hemizonid not seen.
Uterus ~ 70% of total gonad length in uninseminated females, packed with sperm in inseminated females; vagina at right angle to body axis; reproductive tract extending to nerve ring; length of tract hypertrophied in some specimens. Vulva a transverse slit, vulval lips raised ~ 1 µm, no vulval plate present. Anus an obscure pore. Tail sub-cylindroid; relatively short; length 1–1.5 times diameter at anus, tip almost hemispherical.
Male. Body almost straight to arcuate when relaxed by heat, tail region slightly curved ventrally. Cuticle obscurely annulated, longitudinal striae of sub-cuticle apparent when viewed with light microscope; lateral fields not seen.
Cephalic region 5–7µm wide, occupying 75–80% anterior body diameter, offset, ~ 1.5–2 µm high; circum-oral area flat or raised, with lightly sclerotised framework; stylet with cone ~ 40% of length, round stylet knobs 2–3 µm wide. Orifice of dorsal pharyngeal gland ~ 2 µm behind knobs. Anterior fusiform part of digestive tract occupying 52–83% of body diameter, length 2–3 times diameter. Pharyngeal glands extending over intestine, diameter 46–80% (mean 63%) of body diameter, distance from head to end of glands being 32–59% (mean 47%) of total body length. Lumen of intestinal tract broadens behind pharyngeal gland.
Secretory/excretory pore opens opposite nucleus of pharyngeal gland; duct obscure; secretory/excretory cell ovoid, ~ 5 µm long. Hemizonid extending over two annules, 4 or 5 annules in front of secretory/excretory pore.
Reproductive tract with single testis, variable in length, usually extending to distal end of dorsal pharyngeal gland, barely overlapping it in three specimens, not flexed; testis, seminal vesicle and vas deferens not clearly differentiated. Bursa apparently leptoderan, smooth; may be prominent or obscure; arises 40 – 50% along length of body from tail tip, terminates just in front of tail tip. Spicules paired, broadly angular to arcuate, bent at about 40% of length, with manubrium and shaft longer than blade; moderately sclerotised; manubrium wider than shaft, may be off-set on dorsal edge; blade narrows gradually to bluntly rounded tip with concavity on distal edge; opening sub-terminal. Inconspicuous muscles associated with cloaca. Tail arcuate, slightly ventrally concave, conoid; length 1.5–2.5 times diameter at cloaca; bluntly to broadly rounded tip.
Diagnosis and relationships. Fergusobia rosettae n. sp. is morphologically characterized by the combination of a small, arcuate parthenogenetic female having a short conoid tail with a bluntly rounded tip, an arcuate, relatively slender, infective female with an almost hemispherical tail tip, and an arcuate male with an arcuate to angular (not heavily sclerotised) spicule and leptoderan bursa arising at 40–50% of body length.
Morphologically, Fergusobia rosettae n. sp. is similar to F. pohutukawa Davies 2007 (in Taylor et al. 2007), F. jambophila Siddiqi 1986 , F. tolgaensis n. sp., F. cajuputiae Davies & Giblin-Davis 2004 , F. nervosae Davies & Giblin-Davis 2004 , and F. sporangae Davies 2013 (in Davies et al. 2014a).
From phylogenetic analyses based on sequences of D2/D3, large sequence divergences support F. rosett ae n. sp. as a unique species. It is genetically close to an undescribed species ( Fergusobia sp. 1 in Davies & Giblin-Davis 2004) from a similar gall form on M. nervosa ( Ye et al. 2007) .
The parthenogenetic female of F. ro s et t a e n. sp. (arcuate shape) differs from F. brevicauda Siddiqi 1994 , F. brittenae Davies 2010 (in Taylor & Davies 2010), F. cosmophyllae Davies 2013 (in Davies et al. 2013b), F. diversifoliae Davies 2013 (in Davies et al. 2013b), F. fasciculosae Davies 2012 (in Davies et al. 2012b), F. floribundae Davies 2013 (in Davies et al. 2013b), F. gomphocephalae Davies 2013 (in Davies et al. 2014c), F. indica Jairajpuri 1962 sensu Siddiqi 1986 , F. leucoxylonae Davies 2014 (in Davies et al. 2014c), F. magna Siddiqi 1986 sensu Davies 2010 (in Davies et al. 2010b), F. microcarpae Davies 2014 (in Davies et al. 2014b), F. minimus Lisnawita 2013 (in Davies et al. 2013b), F. morrisae Davies 2012 (in Davies et al. 2012b), F. pimpamensis Davies 2013 (in Davies et al. 2013b), F. planchonianae Davies 2014 (in Davies et al. 2014b), F. porosae Davies 2013 (in Davies et al. 2013a), F. ptychocarpae Davies 2008 (in Taylor & Davies 2008), F. tumifaciens ( Currie 1937) Wachek 1955 sensu Davies 2014 (in Davies et al. 2014b), F. v i m i n al i s a e Davies 2014 (in Davies et al. 2014b), and F. viridiflorae Davies & Giblin-Davis 2004 (C-shape). In length (228–269 µm), it is shorter than the parthenogenetic female of F. rileyi Davies 2012 (in Davies et al. 2011a) (310–394 µm). In having cuticle which does not swell upon fixation; it differs from F. jambophila Siddiqi 1986 and F. pohutukawa Davies 2007 (in Taylor et al. 2007) in which it does. Having a distinctly raised circum-oral area separates the parthenogenetic female of F. rosettae n. sp. from those of F. cajuputiae , F. colbrani Davies 2014 (in Davies et al. 2014a), F. delegatensae , F. fisheri Davies & Lloyd 1996, F. leucadendrae Davies & Giblin-Davis 2004 , and F. tumifaciens , in which it is flat or only slightly raised. The stylet (8–10 µm) of this female is longer than in F. curriei Fisher & Nickle 1968 (5–8 µm) and F. juliae Davies 2012 (in Davies et al. 2012b) (5–7 µm); and shorter than in F. camaldulensae Davies 2012 (in Davies et al. 2012a) (11–13 µm), F. schmidti Davies & Bartholomaeus 2014 (in Davies et al. 2014c) (11–14 µm), and F. tumifaciens (19 µm). In having an enormous oesophageal gland ( b’ 1.5–2.2), it is similar to F. quinquenerviae Davies & Giblin-Davis 2004 but lacks the extra lobe or flex found in the gland of the latter. In F. rosettae n. sp., the vulva ( V 83 –93%) is more posterior than in F. nervosae Davies & Giblin-Davis 2004 (81–83%). In having a body behind the vulva that narrows gradually, is arcuate and conoid in shape, with a broadly rounded tip, this female differs from that of F. dealbatae Davies & Giblin-Davis 2004 , F. eugenioidae Davies 2012 (in Davies et al. 2012b), and F. philippinensis Siddiqi 1994 (more slender, arcuate to straight). In length (9–21 µm, mean 14 µm), the tail of the F. rosett ae n. sp. parthenogenetic female is usually shorter than in F. tolgaensis n. sp. (mean 22 µm, range 18–25 µm). This female lacks the broad opening of the stylet aperture present in F. sporangae Davies 2014 (in Davies et al. 2014c). It is morphologically close to but can be separated from the undescribed species of Fergusobia (Species 1) from ‘rosette’ galls on M. nervosa in having the hemizonid at 4–5 vs 6–8 annules in front of the excretory pore (Davies & Giblin-Davis 2004).
The infective female of F. ros e t t a e n. sp. (open C-shape) differs in shape from that of F. brevicauda , F. camaldulensae , F. colbrani , F. curriei , F. delegatensae , F. diversifoliae , F. fisheri , F. leucadendrae , F. leucoxylonae , F. microcarpae , F. quinquenerviae , and F. viridiflorae (arcuate to barely J), and from F. rileyi (almost straight). In length (250–267 µm), it is smaller than the female of F. brittenae (375–550 µm), F. c ol b r an i (369–405 µm), F. cosmophyllae (374–448 µm), F. dealbatae (307–347 µm), F. eugenioidae (438 µm), F. fasciculosae (268–332 µm), F. floribundae (357–450 µm), F. juliae (396–550 µm), F. magna (537–633 µm), F. morrisae (322–395 µm), F. pimpamensis (369–443 µm), F. philippinensis (290–370 µm), F. planchonianae (303–339 µm), F. porosae (277–300 µm), F. ptychocarpae (387–471 µm), F. sporangae (289–353 µm), and F. viminalisae (334–437 µm). The stylet (7–8 µm) of F. rosettae n. sp. is longer than in F. minimus (4–6 µm). It has a subcylindroid tail with a tip that is almost hemisperical, separating it from F. tolgaensis n. sp., in which the tail tip is broadly rounded. It is difficult to morphologically separate the infective female of F. rosett ae n. sp. and F. cajuputiae , although the former tends to be slimmer (respective diameters 22–23 µm and 27–30 µm). Body diameter also separates F. rosettae n. sp. and F. gomphocephalae and F. schmidti (respectively, diameters 22–23 µm vs 26–32 µm and 30–48 µm; ratio a 10.8–11.5 vs 8.6–10.7 and 6.5–10.8). Given the small sample size for the infective female of F. nervosae (n=1), and their morphological similarity to F. rosettae n. sp., it is not possible to separate them.
In shape (arcuate), the heat-relaxed male of F. ro s e t t ae n. sp. differs from those of F. brittenae , F. curriei , and F. fasciculosae (J-shape), F. pimpamensis (J or C-shape), F. diversifoliae , F. juliae , F. magna , F. planchonianae , F. ptychocarpae , and F. viridiflorae (with strongly curved posterior). In length (246–319 µm), it is smaller than the male of F. brevicauda (330–420 µm), F. camaldulensae (383–451 µm), F. delegatensae (350–518 µm), F. diversifoliae (413–459 µm), F. eugenioidae (341–420 µm), F. floribundae (403–570 µm), F. morrisae (347–413 µm), F. pohutukawa (398–469 µm), and F. r i l e yi (378–508 µm). The ratio a (8.5–11.4) is smaller than in males of F. pohutukawa (12.2–15.5). In length (8–9 µm), the stylet is shorter than in F. leucoxylonae (10–13 µm) and tends to be shorter than that of F. tumifaciens (8.5–10 µm). The shape of the tail (barely arcuate with a broadly rounded tip) differs from that of F. philippinensis (truncate tip) and from F. leucadendrae (bluntly rounded tip). Spicule length (14–17 µm) is shorter than in F. colbrani (17–21 µm), F. dealbatae (18–22 µm), F. juliae (20–27 µm), F. quinquenerviae (16–20 µm), F. schmidti (19–25 µm), and F. sporangae (17–25 µm). The bursa arises at ~ 40–50% of body length from the tail tip, differing from F. cosmophyllae , F. fisheri , F. minimus , F. porosae , and F. tumifaciens in which it is shorter (respectively, 12–39, ~ 20, 12–28, 15–33, and less than 20%) and from F. cajuputiae and F. jambophila in which it is longer (more than 50%). In having a raised circum-oral area, this male is separated from those of F. microcarpae and F. nervosae , in which it is flat or less raised. The shape of the spicule differs in F. rosettae n. sp. and F. gomphocephalae and F. viminalisae , being more angular in the latter two species. In size, shape, length of bursa, form of cephalic area and of stylet, the male of F. rosettae n. sp. and F. tolgaensis n. sp. are similar. However, they can be separated on the position of the hemizonid (4–5 vs 2 annules in front of the excretory pore), and the spicule is more slender in F. tolgaensis n. sp.
Etymology. Named after the form (resembling a rosette) of the gall from which the nematodes were collected.
TABLE 2. Measurements (µm) of Fergusobia rosettae n. sp. from ‘ rosette’ galls on M. quinquenervia (mean ± standard deviation; ranges in brackets).
| Holotype partheno- genetic female | Parthenogenetic females | Males | Infective females | |
|---|---|---|---|---|
| N | 15 | 21 | 4 | |
| Length | 263 | 247±11.6 (228–269) | 294±18.0 (246–319) | 258±7.6 (249–267) |
| a | 8.2 | 8.0±0.8 (7.0–10.0) | 9.8±0.9 (8.5–11.4) | 11.3±0.3 (10.8–11.5) |
| b’ | 1.7 | 1.8±0.6 (1.5–2.2) | 2.1±0.4 (1.7–3.1) | 2.9±0.9 (2.1–3.9) |
| c | 18.5 | 18.5±4.7 (11.8–28.0) | 9.6±1.1 (7.3–11.2) | 14.6±0.9 (13.4–15.6) |
| c’ | 1.2 | 1.2±0.3 (0.9–1.7) | 2.0±0.2 (1.7–2.5) | 1.2±0.2 (1.0–1.4) |
| V% | 88 | 88±2.3 (83–91) | 88.7±4.1 (84.8–93.3) | |
| T% | 36.5±13.1 (16–59) | |||
| Body diameter | 32 | 31±2.9 (25–36) | 30±3.5 (23–36) | 23±0.4 (22–23) |
| Stylet length | 8 | 9±0.7 (8–10) | 8±0.5 (8–9) | 8±0.6 (7–8) |
| Ant. end to SE pore | 87 | 73±9.0 (57–87) | 76±5.6 (69–89) | 72±9.5 (64–85) |
| Spicule length | 16.5±1.0 (14–17) | |||
| Tail length | 14 | 14±3.7 (9–21) | 31±3.3 (25–37) | 18±1.5 (16–20) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |