Arsipoda rostrata Gómez-Zurita, 2010
|
publication ID |
https://doi.org/10.1080/00222933.2010.499575 |
|
persistent identifier |
https://treatment.plazi.org/id/117D87F8-350B-EF74-FE75-B14E9D61FB69 |
|
treatment provided by |
Felipe |
|
scientific name |
Arsipoda rostrata Gómez-Zurita |
| status |
sp. nov. |
Arsipoda rostrata Gómez-Zurita sp. nov.
Type material
Holotype: one male, Nouvelle Caledonie: Prov. Sud, Mt Koghi , ca. 600 m, 12–16 November 2001, K.A. Johanson, T. Pape and B. Viklund leg. Voucher no. (specimen and DNA): IBE-JGZ-1062. The holotype is deposited in the Entomology collection of the Naturhistoriska Riksmuseet (Stockholm, Sweden). Left antenna of the holotype is broken, but all segments are mounted together with the specimen.
Description
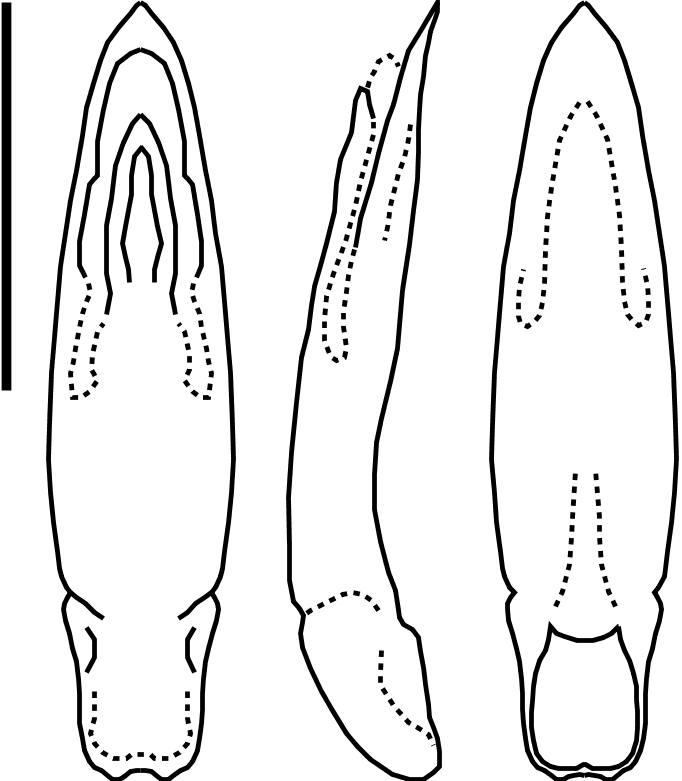
Habitus ( Figure 4B View Figure 4 ). Body elongate oval, 2.43 mm long and 1.07 mm wide, moderately convex; body uniformly brown with hind femora, antennae and frontally on head paler, and last five antennal segments darker; hind femora preapically broadly infuscate; anterior and median legs, metatibiae and metatarsi yellowish. Winged species.
Head. Frons smooth, glossy, unpunctured, with delicate oblique striae behind eyes; surface of vertex slightly irregular, with sparse shallow punctures between eyes; short longitudinal furrow dorsolaterally above and next to eye, proximally ending at small fovea bearing erect seta. Postantennal calli oblique, obsolete. Interantennal space almost flat, narrow (1.33 × transverse diameter of antennal socket). Eyes large, convex, elongated anteroposteriorly (ratio 12: 21.5). Genae long (1.33 × transverse diameter of antennal socket); clypeus depressed at sides, also long, unpunctured, with transverse anterior row of six very fine, whitish setae, two median setae shorter. Labrum transverse, relatively long (0.38 × as long as wide), unpunctured, curved at apical angles, straight anteriorly, with very weak dorsal emargination at apex and two short very fine setae transversally at each side of disc. Maxillary palpi elongated; last segment slightly narrower than preceding, cylindrical, with rounded apex. Antennae long, slender; first segment elongated, gradually expanding to midlength and subparallel-sided apically; second segment elongated ovoid, shorter (0.73 ×) than first; third segment slightly shorter than second, narrower, weakly gradually expanded apically; fourth segment as long as first, club-shaped, clearly wider than third; segments 5 to 10 longer than fourth, subcylindrical, with segments 7 and 8 longest (1.5 × longer than first), slightly granulose and rather pubescent; last segment longest (1.7 × longer than first), tapering at two-thirds to rounded apex.
Pronotum. Trapezoidal, slightly transverse (longitudinal midlength: 0.47 mm), disc weakly convex with sides laterally sloping, strongly on apical half; anterior margin (0.5 mm wide between anterior angles) almost straight, basal margin convex (0.70 mm wide), with slightly protruding median lobe, both very finely bordered; sides straight, with margin feebly explanate laterally bearing four or five rather deep spaced punctures alongside margin internally; anterior angles protruding as obliquely swollen calli with rather large round fovea bearing a single long erect seta; posterior angles straight, rounded, with setigerous pore at angle. Surface of pronotum bright, delicately shagreened, with dense shallow punctures, slightly deeper at sides of disc, each puncture bearing a very fine and small, appressed backwards seta; deep basal transverse, weakly sinuous furrow at about one-quarter of pronotal length, with ends at level with humeral internal depression of elytra, marked by very short longitudinal fold. Proepisterna almost flat, but perceptibly decliving at apex; glossy, very sparsely and finely punctured. Prosternum transversally convex, almost as long as longitudinal section of procoxae; anterior margin concave, very finely bordered, fringed anteriorly by dense long pale yellowish setae; surface smooth, glossy, impunctate, except at small area near anterior angles and six setigerous punctures on median area bearing appressed backwards fine long setae; prosternal process very narrow, as broad as maxillary palp segments, but quickly expanding apically surrounding and enclosing procoxae posteriorly, with apex slightly convex and very finely bordered laterally and apically. Scutellum almost twice as broad basally as long, nearly semicircular, smooth, unpunctured.
Elytra. Long (1.67 mm long, 0.53 mm wide at middle), flattened at disc, regularly convex at lateral declivities and posteriorly; broader than pronotum at round humeral angles; humeral calli protruding, glossy, unpunctured; sides regularly but weakly curved, broadest at middle, gradually converging to broadly rounded apex; finely margined from humeral to sutural angles, with margin slightly explanate, entirely visible from above except shortly below humerus and apical declivity of elytra; surface of elytra glossy, smooth, with very few secondary very minute punctures on intervals and sparse, very fine and minute scattered setae, slightly denser on disc; surface regularly striate/punctate, with punctures becoming unordered, obsolete at apical declivity; scutellar row reaching one-third of elytra, with nine relatively deep punctures; rows 2 to 6 running from base to blurred apical area of elytra, weakly sinuous, more markedly for second row, approaching suture beyond apical end of sutural row; rows 7 to 10 starting behind humeral prominence, running parallel to elytral margin; striae interspace slightly convex, particularly at lateral declivity; margin of elytron regularly punctured. Epipleura broad basally, glossy, unpunctured, thinning gradually, almost disappearing towards apex, laterally oblique, entirely visible from side; inner margin punctured in all its length.
Ventral parts. Mesoventrite concave, apically narrowly subquadrate between mesocoxae with apical angles produced as small teeth. Metaventrite long, rather convex in transverse section, finely bordered, with longitudinal median impression, glossy, unpunctured except for uniformly sparse setigerous punctures bearing very fine translucent appressed backwards setae. Procoxae slightly transverse; mesocoxae swollen but depressed posteriorly to receive retracted femora; metacoxae narrow, transverse, depressed below level of metaventrite. Profemora and mesofemora spindle-shaped, sparsely pubescent; metafemora very strongly developed, greatly enlarged dorso-anteriorly (1.86 × longer than wide), minutely, almost imperceptibly punctured, finely sparsely pubescent, with denser hairs towards apex, longitudinally depressed posterodorsally at apex to receive flexed metatibiae. Pro- and mesotibiae almost straight, gradually expanding towards apex, finely punctured and relatively densely setose; metatibiae straight in lateral view, slightly bent outwards and flattened in dorsal view; small tooth dorsally, near apex of metatibia on external margin
mm
0.5
followed by row of minute spines; apex of metatibiae armed externally with relatively large, darkened spur. First protarsal and mesotarsal segments enlarged, twice as long as broad, and as long as next two segments together; metatarsi narrow, elongated, with first segment 1.33 × longer than next two segments together. Claws appendiculate. Abdominal sternites very convex laterally, very feebly shagreened, pubescent with fine translucent, relatively long setae, except medially on four last segments; last segment longer than two preceding together, apically trilobate, with median lobe depressed and internal marginal ends of lateral lobes slightly superimposed on depressed median area as short ridges, possibly assisting locking mechanism of the genital orifice by the pygidium.
Aedeagus. 3.75 × longer than widest point at slightly less than two-thirds from apex (1.00 mm long, 0.27 mm wide); spearhead-shaped, with sides regularly curved to rather acuminated apex; dorsally presenting a large elongated membranous operculum covered by a chitinous lancet-like ligule; straight in lateral view, slightly bent basally at level with widest point on ventral view ( Figure 7 View Figure 7 ).
Diagnosis
This small and stylized species of Arsipoda could be confounded at first sight with the two other slender New Caledonian species in the genus with straight or slightly concave sides of the pronotum, namely A. shirleyae and A. isola . However, A. rostrata Gómez- Zurita sp. nov. has several remarkable diagnostic traits, including the darker, more reddish-brown upper body coloration, a more elongated head, the punctation on elytra slightly sparser (for instance it bears nine punctures on sutural row compared with 13 or 14 in the other species), but chiefly on the shape of the aedeagus, which unequivocally differentiates these species. The aedeagus in A. shirleyae has a broadened apex with a small median apical tooth as opposed to a gradually tapering pointed end in the new species. That of A. isola , although it is also lancet-like, is narrowed in the middle and slightly broadened apically, not widest postmedially and gradually narrowing towards apex as in A. rostrata Gómez-Zurita sp. nov., besides showing an apically expanded ligule.
Distribution
Endemic to New Caledonia.
Etymology
The holotype of this species has a particularly long head, owing to an elongated rostrum, hence the Latin adjective (fem.) for beaked, curved at the end, or rostrated, rostratus, - a, - um.
Ecology of New Caledonian Arsipoda
The information regarding the ecology of the genus Arsipoda is scarce. Different species have been reported as feeding on several plant tissues, including leaves, flowers and, most remarkably, pollen of several plant families. Host records exist for the Australian A. concolor on Juncaceae ( Hawkeswood and Furth 1994) , Arsipoda holomelaena (Germar) on Hemerocallidaceae ( Duncan et al. 2004) , and Arsipoda chrysis (Olivier) on Asteraceae ( Bruzzese 1996) , and the Solomon’s Arsipoda salomonensis Bryant , the southeast Asian A. tenimberensis (Jacoby) and the Australian Arsipoda parvula Jacoby on Convolvulaceae ( Samuelson 1967; Kimoto et al. 1984; Hutton et al. 2008); the Solomon’s endemic was also recorded on Araceae ( Bryant 1941) . Some New Caledonian species were found on Ericaceae and Proteaceae in the case of A. evax , Cunoniaceae , Myrtaceae , Phellinaceae and also Proteaceae in the case of A. isola , and Winteraceae and Anacardiaceae , as well as Phellinaceae , for several unidentified species of Arsipoda ( Samuelson 1973, 1989). Most interestingly, some of these records, particularly those for Anacardiaceae , Phellinaceae and Winteraceae , but also Proteaceae , were confirmed for pollen consumption, which is a reportedly rare feeding strategy in leaf beetles ( Samuelson 1989, 1994). In any case, the trophic spectrum for New Caledonian Arsipoda reveals a relatively low degree of specialization, or an opportunistic behaviour, certainly for flower consumption, with the same species able to change diet even along temporal or altitudinal gradients ( Samuelson 1994).
Two-thirds of beetle specimens checked for the presence of chloroplast DNA sequences in their total DNA extracts either failed to amplify anything or produced complex band patterns on the gel. This may be in great part related to the relatively low DNA quality obtained from specimens that remained dead on Malaise traps, maybe for days, in tropical weather before finally being recovered and transferred into absolute ethanol. Another possible explanation, particularly for examples producing unspecific amplifications, may be related to the expected mixed diet of these animals. However, one-third of the specimens tested produced single band patterns that yielded high quality trn-L (UAA) intron sequences. One of the females of A. geographica Gómez-Zurita sp. nov. produced one trn-L (UAA) intron sequence (EBI acc. no. FN773527) sister to that of the species Ardisia speciosa (posterior probability
Araucaria Pinaceae C Pieris Gaylussacia
Arcterica Andromeda Vaccinioideae
1058
1057 A. speciosa Ledum Menziesia Rhododendron
Rhododendron Rhododendron Ericoideae
Myrsinaceae 0.93
(pp) = 0.86), within a clade of Ardisia (pp = 0.85), a genus of Myrsinaceae related to the generotypic taxon of the family, the genus Myrsine (pp = 0.86) ( Figure 8B View Figure 8 ). The food plant inference can be clearly restricted to the Myrsinaceae of the Ericales . Myrsinaceae is a diversified family in New Caledonia, with species in the genera Maesa (1), Rapanea (14) and Tapeinosperma (39) ( Jaffré et al. 2004; Schmid 2006).
Ardisia has been recognized as an important pest in many Indian and Pacific islands, but so far has been prevented from invading New Caledonia (e.g. Meyer et al. 2006; Soubeyran 2008). The absence of Ardisia in Grande Terre and the recovery of this Ardisia -like sequence from a phytophagous beetle collected in a remote and isolated area of the island raise the question of a potentially undiscovered myrsinaceous plant and Arsipoda host in the Special Reservation for Fauna and Flora of the Kouakoué.
A specimen of A. isola (1065) from Monts Kwa Ne Mwa, even if captured from a trap, yielded a clear sequence (EBI acc. no. FN773528 View Materials ) very closely related to the only available homologous sequence of Pinus pinaster in GenBank (pp = 1.00) ( Figure 8A View Figure 8 ). This is a problematic result in several respects. First of all, unlike most angiosperms, gymnosperm pollen carries chloroplast DNA, which makes it a potential contamination source for this molecular technique, considering that this type of pollen is almost ubiquitous. Besides, contamination is more likely to affect samples of low DNA quality, as expected for the specimens at hand.
Second, there are no native species of Pinus in New Caledonia. Nevertheless, the introduction of Pinus caribaea in the island for commercial use in 1966 is well documented ( Crémière and Ehrhart 1990), and most remarkably that of P. pinaster in the Nouméa region in 1900, although not for economic purposes ( MacKee 1985). The sequence of P. caribaea was not available for analysis, but it belongs to the section Trifoliae of the subgenus Pinus , together with other representatives included in the trn-L (UAA) phylogeny, which none the less did not attract the hypothetical diet sequence to their vicinity, whereas P. pinaster and Pinus canariensis (both in the section Pinus ) did with high support. In any case, considering the pollinivorous and arguably polyphagous nature of these animals, although sample contamination is the most likely explanation for this result, finding gymnosperm remains in their diet falls within the possible range of purely biological explanations as well (see below).
Finally, another A. isola specimen, this time from Plaine des Lacs and freshly collected, produced a sequence (EBI acc. no. FN773526) clearly belonging in the family Ericaceae (pp = 0.89), and sister to the available representatives of the subfamily Vaccionioideae (pp = 0.85). In this case, fixing the number of GenBank hits to 100 as in our default procedure did not allow finding the relevant plant family node for the diet sequence; increasing it to 250 clearly showed its placement within Ericaceae and related to Vaccionioideae ( Figure 8C View Figure 8 ). The flora of New Caledonia only includes two known native Ericaceae in this same subfamily, the species Paphia neocaledonica and Paphia paniensis ( Jaffré et al. 2004; Venter and Munzinger 2007). Interestingly, both species have very restricted ranges in the Plateau de Dogny and Mont Panié, respectively, so relatively far away from the collecting site of the beetle specimen. This situation also raises the question whether there is yet another undiscovered ericaceous plant in New Caledonia, an idea supported by the phylogenetic position of the purported diet sequence from the beetle, basal to Vaccinioideae and removed from the available representatives of the tribe Vaccinieae , which includes Paphia and the related genus Agapetes ( Kron et al. 2002) .
The results obtained for A. isola associated with P. pinaster need to be interpreted with some caution with the preliminary information at hand, but those from the other specimens can be put into a reliable ecological context. Our empirical data obtained from plant remains in the beetle DNA extractions reveal two different food choices, specifically on Myrsinaceae and Ericaceae , respectively, both belonging to the Ericales ( Figure 8B,C View Figure 8 ). The association of New Caledonian Arsipoda with Ericaceae had been proposed already, not so in the case of the other family. But, as described above, and supported by our observations, the food choice for these species is extraordinarily broad, even within a single species, and so far covers representatives of seven plant orders.
This ecological versatility has been noted already in the ability of an unidentified species to feed on mango flowers, when this is a plant that was recently introduced in New Caledonia for commercial purposes ( Samuelson 1994). If confirmed, the identification of an introduced gymnosperm as food resource for Arsipoda would be a remarkable finding, expanding the trophic potential even across Spermatopsida or the seed plants, for a group already known to use a huge spectrum of the angiosperms, the flowering plants.
Notes on the distribution of New Caledonian Arsipoda
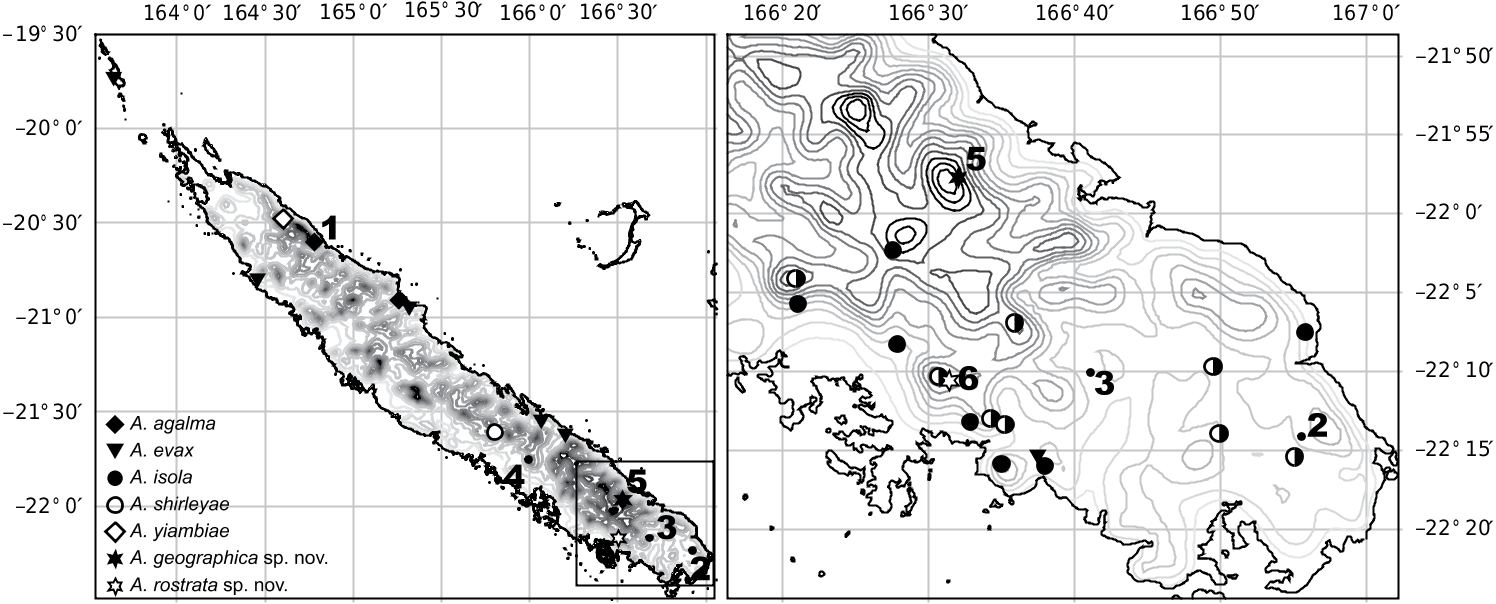
The genus Arsipoda includes species distributed throughout the geography of Grande Terre, from low to high altitudes, and from sclerophyll and rainforests to the maquis minier ( Samuelson 1973). This ample distribution, which may be a reflection or another indication of the ecological versatility discussed above, seems to be true as well for some of the species ( Figure 1 View Figure 1 ). Arsipoda evax has been found from as far south as the environments of Mont-Dore, to one of the Belep Islands, an archipelago of small islets about 50 km offshore from the northwestern tip of Grande Terre. In turn, A. isola and A. shirleyae have been found, often in sympatry as in our samples from Pic Mourrange, in the drier southern third of the island, suggesting more restricted ranges. As for A. agalma and A. yiambiae , they were exclusively known from their type localities on the eastern mountainous tropical part of the island, in the environment of Massif du Panié.
Our sample of A. agalma indeed comes from the type locality, but this is not the case for our specimens of A. yiambiae , collected together with an unidentified species more than 150 km southeast of the type locality, in the also mountainous range of Mont Do. The two new species described here are only known so far from their respective type localities: one already prospected in the past, Mt Koghi not far from Nouméa ( A. rostrata Gómez-Zurita sp. nov.), but the other from a very isolated point in the Massif du Humboldt, very remarkable from a biological point of view and clearly understudied faunistically, the Massif du Kouakoué ( A. geographica Gómez-Zurita sp. nov.).
Concluding remarks
In one of the most recent attempts to quantify the terrestrial animal diversity in New Caledonia, some 4500 known species were recorded, estimating that a five- to fifteen-fold increase in actual species numbers could be expected ( Chazeau 1993). Indeed, over the past 15 years many new taxa have been added to prove how far we are from reaching the ultimate goal of inventorying the whole of the New Caledonian diversity. A keyword-based search in Zoological Records (14 October 2009) for publications since 1993 including the words “ New Caledonia ” and “ Coleoptera ” yielded 47 taxonomic publications with 231 new species descriptions, an increase of 15% over the estimated figure by Chazeau (c. 1500; 1993). In relatively betterknown zoological groups, for example arachnids and reptiles, the same search strategy produced 27 taxonomic papers and 140 new species in the case of ‘Arachnida’ and 23 papers describing 41 species in the case of ‘Reptilia’, in both cases doubling the number of species known in 1993.
In this work we focus on a single genus of a leaf beetle subfamily, the Alticinae, including 11 genera and some 27 species in New Caledonia (Jolivet et al. in preparation). A relatively small sampling obtained from a variety of collecting techniques not necessarily aiming at flea beetles showed that it included new species increasing at least 30% the known species diversity of Arsipoda . Our approach using molecular markers to characterize the animals allowed us, as non-specialists in this group, to quickly recognize the new species, directing our efforts to these new entities for their formal description, while the use of xenobiotic markers also helped in identifying some aspects of the interaction of these animals with their environment, in particular pointing at potential hosts. Interestingly, the inference of plant hosts compatible with the New Caledonian flora yet not covered by the current catalogues, hinted in turn to some undiscovered botanical diversity, this time using an indirect approach. Even though we are aware of New Caledonia bearing a higher Alticinae diversity than currently characterized, with seven species, Arsipoda stands out already as a particularly species-rich genus of flea beetles in the island. It was beyond the scope of this study to prove the monophyly of New Caledonian Arsipoda , but it is likely that it constitutes a natural group, radiating in isolation, as has occurred for several animals and plants (e.g. Eibl et al. 2001; Smith et al. 2007). According to our molecular clock estimates it would also be a relatively recent addition to the island fauna, in the mid-Miocene. The data available on the distribution and biology of these species suggest that in general there are no geographical or ecological (or a mixture of both, e.g. altitude) barriers to dispersal, therefore the high species richness remains intriguing, at least under allopatric or host-shift models of speciation. Alternatively, this species assemblage richness might be the result of several independent colonization events in the Miocene or later, as observed for other insect groups ( Balke et al. 2007; Grandcolas et al. 2009). The presence on the island of several species, independently of them being the result of single or multiple colonization events, may be favoured by their eclectic nature at least regarding food choice. The singularity of the flora of New Caledonia may condition the success rate of herbivorous newcomers to finding an appropriate niche to thrive, unless they are generalists or versatile in behaviour and/or metabolism, as has been shown in the case of Arsipoda , able to use new resources, such as introduced plant species ( Samuelson 1994). This same versatility may prove an advantage against extinction, given the alarming rates of loss of original flora in New Caledonia, currently supporting for instance only 2% of its original tropical dry forest ( Myers et al. 2000; Gillespie and Jaffré 2003). Understanding both the origins and speciation patterns in this genus will require a thorough phylogenetic examination of Arsipoda from neighbouring areas.
This study highlights the importance of investing in fieldwork on biodiversity hotspots as allowed by funding sources. Most importantly, it prompts us to design fieldwork with the appropriate strategies to allow the implementation of molecularbased approaches to taxonomy and the analysis of biodiversity, both from a cataloguing point of view and for the study of biotic interactions. The investigation of these isolated communities, lacking the framework of the broader evolutionary lineage into which they belong, generates perhaps more questions than answers about their evolution, but clearly constitutes the first step towards more extensive approaches, with a wealth of evolutionary hypotheses to test.
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.