Amphelictogon subterraneus bahamiensis Chamberlin, 1918
|
publication ID |
https://doi.org/10.5281/zenodo.156828 |
|
DOI |
https://doi.org/10.5281/zenodo.6275850 |
|
persistent identifier |
https://treatment.plazi.org/id/132EE018-E817-FC51-FEF9-3F0BFC0FF975 |
|
treatment provided by |
Plazi |
|
scientific name |
Amphelictogon subterraneus bahamiensis Chamberlin, 1918 |
| status |
|
Amphelictogon subterraneus bahamiensis Chamberlin, 1918 View in CoL
Figs. 13 View FIGURES 1 3
Amphelictogon bahamiensis Chamberlin, 1918:231 View in CoL 232. Attems, 1938:160 161. Loomis, 1941:35 36, pl. 4, fig. b.
Amphelictogon bidens Loomis, 1934:29 View in CoL 30, figs. 14a, b, pl. 1, fig. 4. Attems, 1938:161. Amphelictogon subterraneus bahamiensis: PérezAsso, 1996 View in CoL , figs. 13AB, 14. Hoffman, 1999:282.
Type specimens. Male holotype of A. bahamiensis ( MCZ) collected by O. Bryant in August 1904 at an unspecified site on Mangrove Cay, Andros Island, Bahamas. Male holotype and female paratype of A. bidens ( NMNH) collected by H. F. Loomis on 4 January 1932 near Arthur’s Town, Cat Island, Bahamas; the original description ( Loomis 1934) mentions two females, but there is only one in the sample today. Mr. Loomis was a participant on the Allison V. Armour Expedition to the West Indies, Guyana, and Surinam (formerly British and Dutch Guiana).
Diagnosis. Prefemoral process and acropodite extending anteriad for equivalent lengths before curving strongly dorsomediad, distal extremity of former smooth and entire, without teeth, not apically bifid.
Description. Body relatively short and slender but typical for family in being comparatively long in proportion to width, sides extending backwards in subparallel arrangement; adult lengths varying from around 1528 mm, maximum widths 2.23.5 mm ( Chamberlin 1918; Loomis 1934, 1941; PérezAsso 1996).
Head with epicranial suture distinct, terminating in interantennal region. Genae narrowly rounded, extending well beyond adjacent cranial margins. Antennae reaching back to caudal margin of 3rd tergite, 1st antennomere short and subglobose, 26 clavate, 7 short and truncate with four terminal sensory cones, relative lengths of antennomeres 2>3=4>6>5>1>7. Facial setae as follows: epicranial 22, supraantennal 22, interantennal 11, subantennal 11, frontal about 66, scattered irregularly across surface, genal 33, clypeal about 77, labral about 1010.
Dorsum glabrous. Collum narrower than succeeding segment, barely overlapping epicranium. Paranota only slightly declined, interrupting slope of dorsum and extending subparallel to substrate, margins angling caudolaterad and extending beyond caudal margins of metaterga, caudolateral corners acutely produced; peritremata moderately thickened, ozopores located near midlength, opening laterad. Epiproct narrow but blunt, with four long apical hairs and four to five pairs of long marginal or submarginal hairs proximad.
Sternum of segment 4 with two rounded, hirsute lobes between anterior legs; postgonopodal sterna shallowly depressed, with three to five pairs of hairs. Gonapophyses in form of short, rounded, indistinct knobs on coxae of 2nd legs. Legs relatively long and slender, without modifications, tarsal claws gently curved. Hypoproct short and inconspicuous, prolonged in midline and apically pointed; paraprocts with margins distinctly thickened and two pairs of long submarginal hairs.
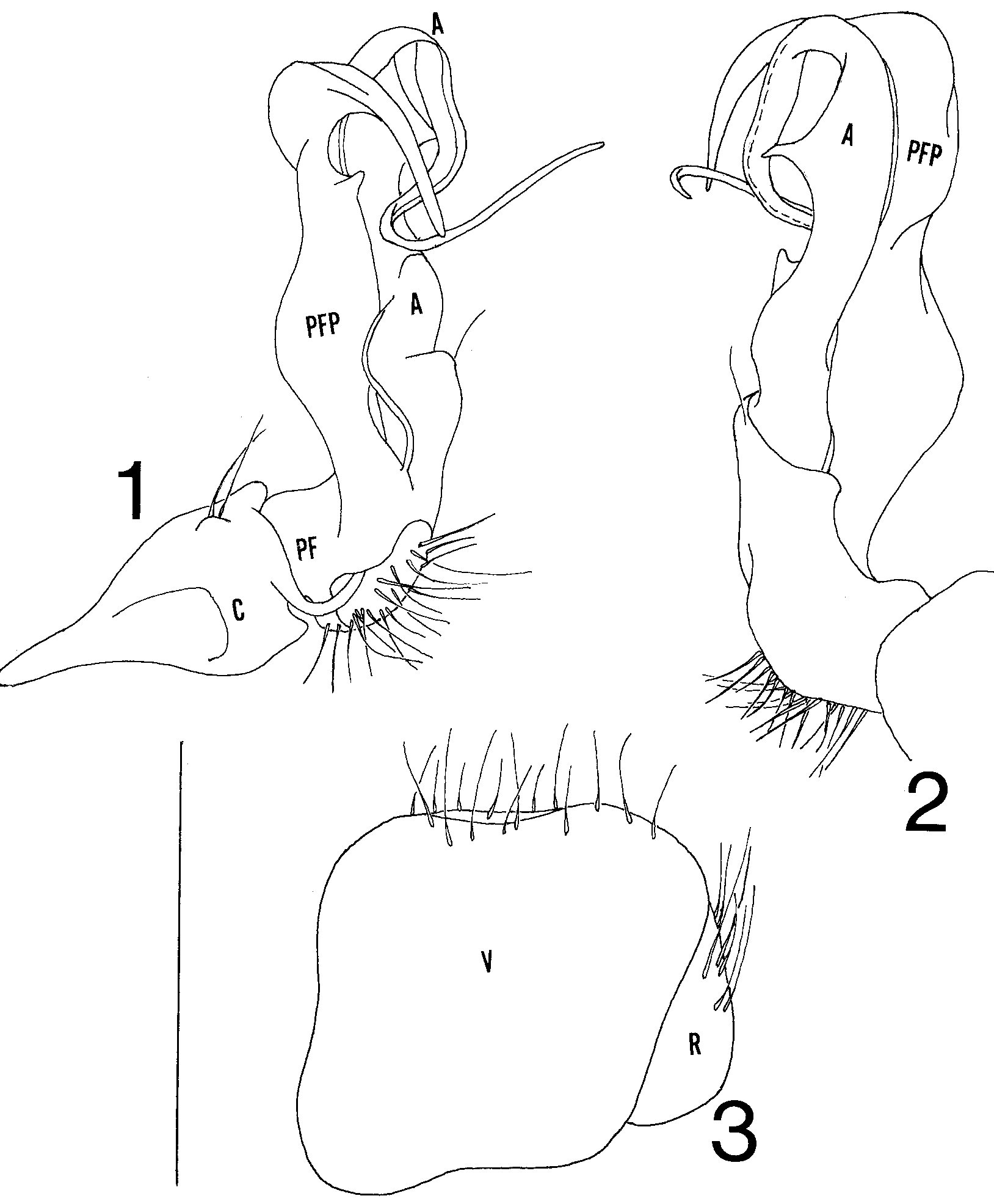
Gonopodal aperture broad, rounded, and obchordate, extending caudad between 9th legs nearly to caudal edge of segment, sides and caudal margin strongly elevated above metazonal surface, anteriolateral corners slightly indented. Gonopods in situ with telopodites extending anteriad over anterior margin of aperture, apices of prefemoral processes and acropodites curving dorsomediad and extending across midline, overlapping opposite member or intertwining. Gonopod structure as follows ( Figs. 12 View FIGURES 1 3 ): coxa and prefemur small, former with rounded distal lobe. Prefemoral process long and complex, extending directly anteriad, sides widening and narrowing with broadly subtriangular spur on anterior margin at 2/3 length, narrowing thereafter and curving strongly dorsomediad, sides narrowing and tapering to smooth, blunt tip. Acropodite extending anteriad for equivalent distance to prefemoral process, with basal shelf, short blunt spur on medial surface at 1/4 length, and spiniform process on anterior margin at ½ length, curving strongly dorsomediad thereafter with sides narrowing continuously into flagelloid prolongation, latter passing through one coil and continuing mediad to acuminate termination. Prostatic groove running along caudal side of acropodite basally, crossing to anterior margin at dorsomedial curve, opening apically.
Cyphopods ( Fig. 3 View FIGURES 1 3 ) minute, oriented obliquely in aperture. Valves subequal and subrhomboid, with a few scattered hairs along ventral margins. Receptacle located on dorsomedial side of valves, with long ventral hairs. Operculum not detected.
Var ia t io n. In addition to being fragmented, the Eleuthera specimen was rigid, darkened, and discolored after over a century in alcohol; there was no evidence of the original pigmentation pattern as was also the case with the type of A. bahamiensis ( Chamberlin 1918) . PérezAsso (1996, fig. 14) discussed the extraordinary color variation in this species and illustrated 38 variants.
The acropodite tapers into a fragile, flagelloid prolongation that normally coils through a complete loop at the level of the tip of the prefemoral process before continuing outward to the termination. The coils of opposing acropodites may overlap or intertwine, requiring one to untangle them during dissection; it is thus easy to disrupt this coiled configuration as happened in fig. 1.
Ecology. Habitat is not provided on vial labels for the Andros and Eleuthera samples, nor did Chamberlin (1918) mention it for the former. According to Loomis (1934), the Cat Island sample was discovered in a natural pit in limestone, referred to locally as a “banana hole” because banana trees are planted in them to provide protection from the winds. Loomis also noted that humus layers in the Bahamas are sparse because the islands are low and consist primarily of porous limestone that drains rapidly; moreover, rains are seasonal, droughts are frequent, and sea breezes cause rapid surface evaporation. Consequently, vegetation is rarely dense enough to support the accumulation of substantial humus layers, and pits offer the most favorable habitat for moisture requiring soil arthropods.
Distribution. Known from Cayo Coco, Cuba, and single localities apiece on Andros, Cat, and Eleuthera islands, Bahamas ( Fig. 4 View FIGURE 4 ). Hoffman (1999) implied that the milliped occurs throughout the Archipiélago de Camagüey, but the only localities that I am aware of are from Cayo Coco specifically. Details of the new sample are as follows:
BAHAMAS: Eleuthera I., Tarpum Bay,, 17 November 1890, collector unknown (AMNH).
Remarks. Amphelictogon subterraneus bahamiensis is the only Bahamian representative of the family Chelodesmidae and the suborder Leptodesmidea. Hoffman (1999) suspected that the Andros record was inauthentic, but with samples now known from three different islands, it is unlikely that they all reflect human agency or mislabeling. I believe they represent genuine Bahamian populations that may be small and possibly no longer exist, particularly on Andros Island, which is covered by extensive pine forests that typically harbor few millipeds. Andros, Eleuthera, and Cat islands are centrally located in the archipelago and are coherent with regards to Cayo Coco ( Fig. 4 View FIGURE 4 ), so their populations conceivably arose by rafting, particularly since hurricanes uproot trees and deposit substantial debris into the waters off Cuba nearly every year. Andros is around 133 mi ( 213 km) due north of Cayo Coco, within plausible rafting distance; Cat and Eleuthera islands, only 15 mi ( 24 km) apart, are more distant, ca. 209 and 230 mi (334 and 368 km) from Cayo Coco, but individuals could have subsequently rafted from Andros to these two islands, some 100 and 120 mi (160 and 192 km) to the east, though today it would require a circuitous route to avoid running aground on the intervening islands and reefs. However, when sea levels were lower during the Pleistocene, the present Bahamian Archipelago was reduced to five “superislands” that correspond to the present “banks” shown on maps; Andros, Eleuthera, and Cat islands were part of the irregularly shaped largest island represented by “Great Bahama Bank,” which also included Long Island, the Exumas, New Providence, and Bimini ( Fig. 4 View FIGURE 4 ). Cuba too was larger, and the present Archipiélago de Camagüey was fused with the main island; the shortest distance across the “Old Bahama Channel” between these two ancient land masses was where the Camagüey islands are today and was only about 20 mi ( 32 km), a much more feasible distance for a rafting event to traverse. Rafting from Cuba and possibly also the Bahamas is considered the most likely explanation for the populations of the centipede, Scolopendra alternans Leach, 1813 , in Collier, Dade, and Monroe counties, Florida ( Shelley 2002 b), and Roth and Bogan (1984) concluded that rafting was the only plausible explanation for the occurrence in south Florida of the Cuban tree snail, Liguus fasciatus (Müller) . Likewise, rafting from the coasts of Ecuador and Peru, a much greater distance than that postulated here, is considered the most likely explanation for the occurrence of the centipede, Scolopendra galapagoensis Bollman, 1889 , in the Galápagos Archipelago (Shelley and Kiser 2000). Additionally, Pereira et al. (1999) reported that the centipede, Pectiniunguis halirrhytus (Crabill, 1959) , which has only been found on beaches in association with seaweed and “beach drift,” some below the high tide line, occurs in both the lower Keys of Florida and Cozumel and Quintana Roo, Mexico, implying that rafting is also operative in its dispersal. It is therefore reasonable to hypothesize that the Bahamian populations of A. s. bahamiensis arose during the Pleistocene by rafting the short 20 mi ( 32 km) distance between the Cuban and “Great Bahama Bank” land masses that existed at that time. The milliped then dispersed through the latter ( Fig. 4 View FIGURE 4 , arrows), and the present populations represent fragments of this single Pleistocene population that became isolated on the three known islands as sea levels rose during the postPleistocene era. The available samples were taken from 71 to 113 years ago, so Andros, Eleuthera, and Cat islands warrant investigation to determine if this unique component of the Bahamian arthropod fauna still survives. An extant population on Cat Island seems plausible, because it was formerly covered with hardwoods and there is permanent moisture and decomposing litter around the entrances to caves. The other islands that were once joined into this “superisland” also warrant investigation, particularly Long Island, Great Exuma, and New Providence, which lie between presentday Andros and Eleuthera/Cat islands on Great Bahama Bank.
The Bahamian samples of A. s. bahamiensis are further significant in being the northernmost for the subfamily Chelodesminae and the only ones occurring north of the Tropic of Cancer; the Eleuthera site specifically is the northernmost for the subfamily, genus, species, and subspecies. The only chelodesmid occurring farther north is the prepodesmine Cantabrodesmus lorioli Mauriès, 1971 , from Cueva del Molino, Santander Province, Spain ( Mauriès 1971, 1974; Hoffman 1980; Shelley et al. 2000), which is known only from the holotype and paratype that were collected in 1961.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Leptodesmidea |
|
Family |
|
|
Genus |
Amphelictogon subterraneus bahamiensis Chamberlin, 1918
| Shelley, Rowland M. 2003 |
Amphelictogon bidens
| Attems 1938: 161 |
| Loomis 1934: 29 |
Amphelictogon bahamiensis
| Loomis 1941: 35 |
| Attems 1938: 160 |
| Chamberlin 1918: 231 |