Macrobiotus azzunae, Marnissi & Cesari & Rebecchi & Bertolani, 2021
|
publication ID |
https://doi.org/10.5852/ejt.2021.758.1429 |
|
publication LSID |
lsid:zoobank.org:pub:C4F57584-0AD9-47C9-BA95-02133C434941 |
|
DOI |
https://doi.org/10.5281/zenodo.5092922 |
|
persistent identifier |
https://treatment.plazi.org/id/933CCC06-F69D-49E2-AF4F-0C042D8F5C99 |
|
taxon LSID |
lsid:zoobank.org:act:933CCC06-F69D-49E2-AF4F-0C042D8F5C99 |
|
treatment provided by |
Felipe |
|
scientific name |
Macrobiotus azzunae |
| status |
sp. nov. |
Macrobiotus azzunae View in CoL sp. nov.
urn:lsid:zoobank.org:act:
Figs 1–4 View Fig View Fig View Fig View Fig , 5A, C View Fig , 7 View Fig
Etymology
The new species is dedicated in honor of Atf Azzouna, professor in the Faculty of Mathematical, Physical and Natural Sciences of Tunis and supervisor of the PhD thesis of Jamila Ben Marnissi.
Type material
Holotype TUNISIA • spec. of unidentified sex; North-West Tunisia, Kroumiri Mountains, Ain Soltan forest , Jendouba; 36º31′21.788″ N, 8º19′57.741″ E; 893 m a.s.l.; Apr. 2017; Marnissi leg.; moss on trunk of Quercus canariensis ; UNIMORE, slide code C4218–S32. GoogleMaps
Paratypes
TUNISIA • 17 specs, sex unidentified; same collection data as for holotype; UNIMORE, slide codes C4218–S2 to C4218–S7, C4218–S9, C4218–S17, C4218–S30, C4218–S31, C4218–S33 to C4218– S35 GoogleMaps • 3 eggs; same collection data as for holotype; UNIMORE, slide codes C4218–S10, C4218–S11, C4218–S25 GoogleMaps .
Type depositories
The holotype (slide: C 4218– S 32), 17 paratypes (slides C 4218– S 2 to C 4218– S 7, C 4218– S 17, C 4128– S 30, C 4218– S 31, C 4218– S 33 to C 4218- S 35), 3 eggs (slides: C 4218– S 10/11/25) and two vouchers (slides SP 04 and SP 07, corresponding to specimens C 4218 V 4 and C 4218 V 7, respectively) mounted in Faure-Berlese fluid, are deposited in the Bertolani collection at the Department of Life Science, UNIMORE, Modena, Italy.
Type locality
NW Tunisia, Kroumrie mountains , Ain Soltan forest , Jendouba, 36º31′21.788″ N, 8º19′57.741″ E. Altitude 893 m a.s.l GoogleMaps .
Description
Adult specimens
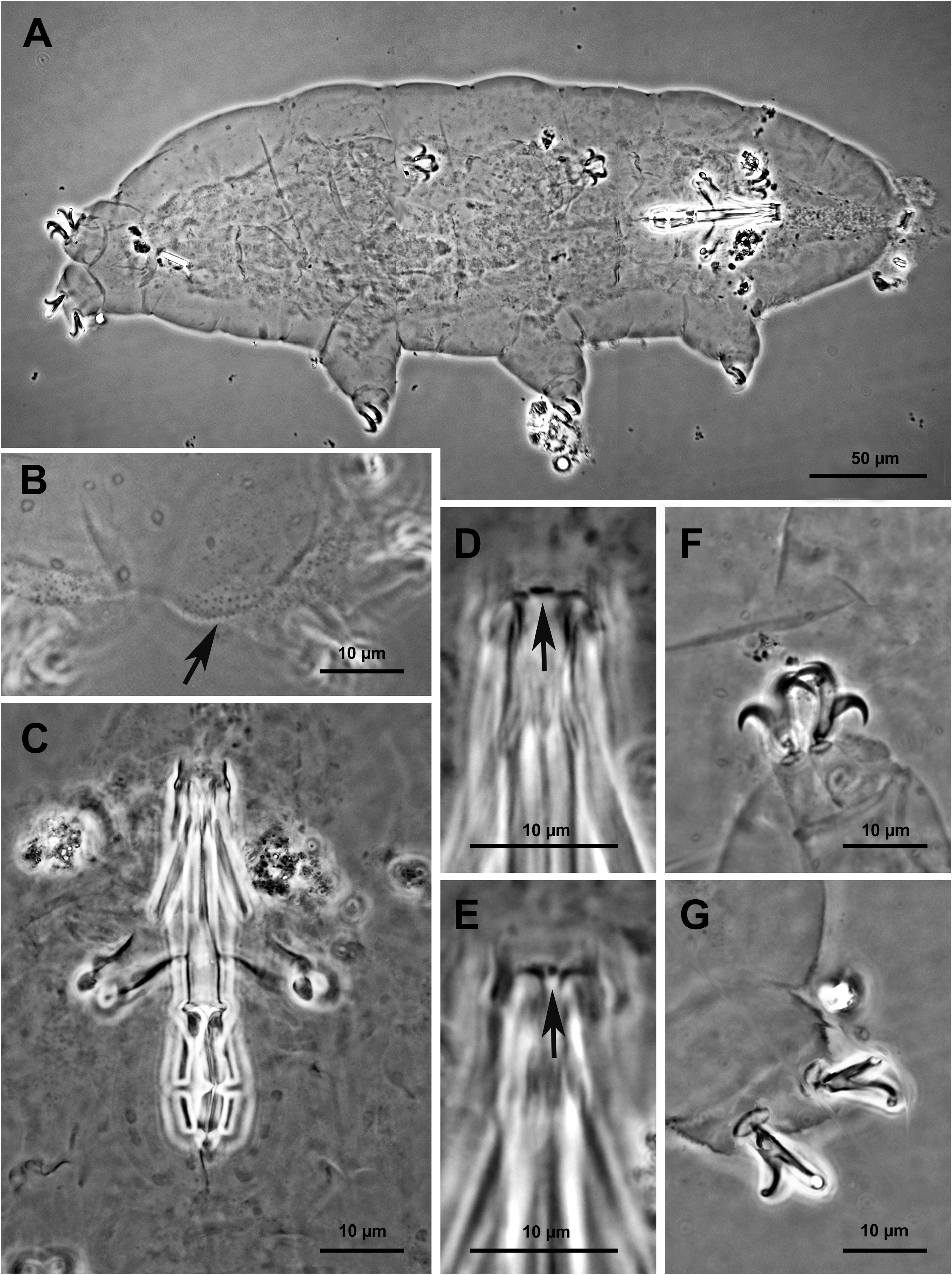
Body white, transparent after mounting in Faure-Berlese, from 162.2 to 410.3 µm in length ( Fig. 2A View Fig , Table 3 View Table 3 ; structures measured only in the animals more than 200 µm in length). Eye spots present, even after mounting. Cuticle smooth but with small round or oval pores, 1–1.5 µm in diameter ( Fig. 2B View Fig ), better visible after fixation in Carnoy and orcein staining ( Fig. 3C View Fig ), scattered randomly on the entire cuticle, including the dorsal surface of all legs. With SEM, pores look oval or in the shape of a seed ( Fig. 3A, D View Fig ) with the largest diameter of 0.7–0.8 µm. Weak cuticular granulation also present on the lateral surface of all legs and specially on legs IV ( Fig. 2B View Fig , arrow). Only with SEM is it possible to define the shape of the granulation on the legs, which looks as a regular disposition of star-shaped protuberances (about 0.3 µm; Fig. 3F View Fig ). Six buccal sensory lobes around the mouth, well recognizable with SEM. Mouth antero-ventral; buccal-pharyngeal apparatus of the Macrobiotus type (sensu Pilato & Binda 2010), with ventral lamina and ten small peribuccal lamellae (in the holotype, after mounting, separated from the mouth). Buccal armature, corresponding to oral cavity armature, OCA, according to Michalczyk & Kaczmarek (2003), without an anterior band of teeth visible, corresponding to the first band of teeth according to Michalczyk & Kaczmarek (2003), and to the anterior band of the buccal ring according to Guidetti et al. (2012); posterior band of teeth poorly visible, corresponding to second band of teeth, according to Michalczyk & Kaczmarek (2003), followed by three dorsal and three ventral crests, corresponding to third band of teeth according to Michalczyk & Kaczmarek (2003); the dorsal crests ( Fig. 2D View Fig ) are distinct transverse ridges, whereas the ventral crests ( Fig. 2E View Fig ) appear as two separate lateral transverse ridges and a roundish median tooth. The posterior band of teeth and the transverse ridges are part of the buccal tube, according to Guidetti et al. (2012). Buccal tube narrow; pharyngeal bulb spherical with triangular apophyses, two rod-shaped macroplacoids, relatively short, the first longer than the second and evidently but not deeply narrowed at its middle ( Fig. 2C View Fig ), the second with a not particularly evident subterminal constriction. Microplacoid present. Slender claws of the hufelandi type (sensu Pilato & Binda 2010); the external claw longer than the internal one and the posterior longer than
the anterior. Primary branches of each claw with distinct accessory points ( Fig. 2F View Fig ), a common tract of medium length (about a third of the total claw length) and an evident stalk connecting the claw to the lunule. Lunules under all claws, smooth, larger on the hind legs ( Figs 2G View Fig , 3E View Fig ). Cuticular bars under claws absent.
The population is dioecious (gonochoristic). Males were recognized using orcein staining, which revealed that the testis is filled with spermatozoa with a coiled head ( Fig. 3G View Fig ) and spermatids. No morphological secondary sexual dimorphism, such as gibbosities on legs IV in males, was identified.
Eggs
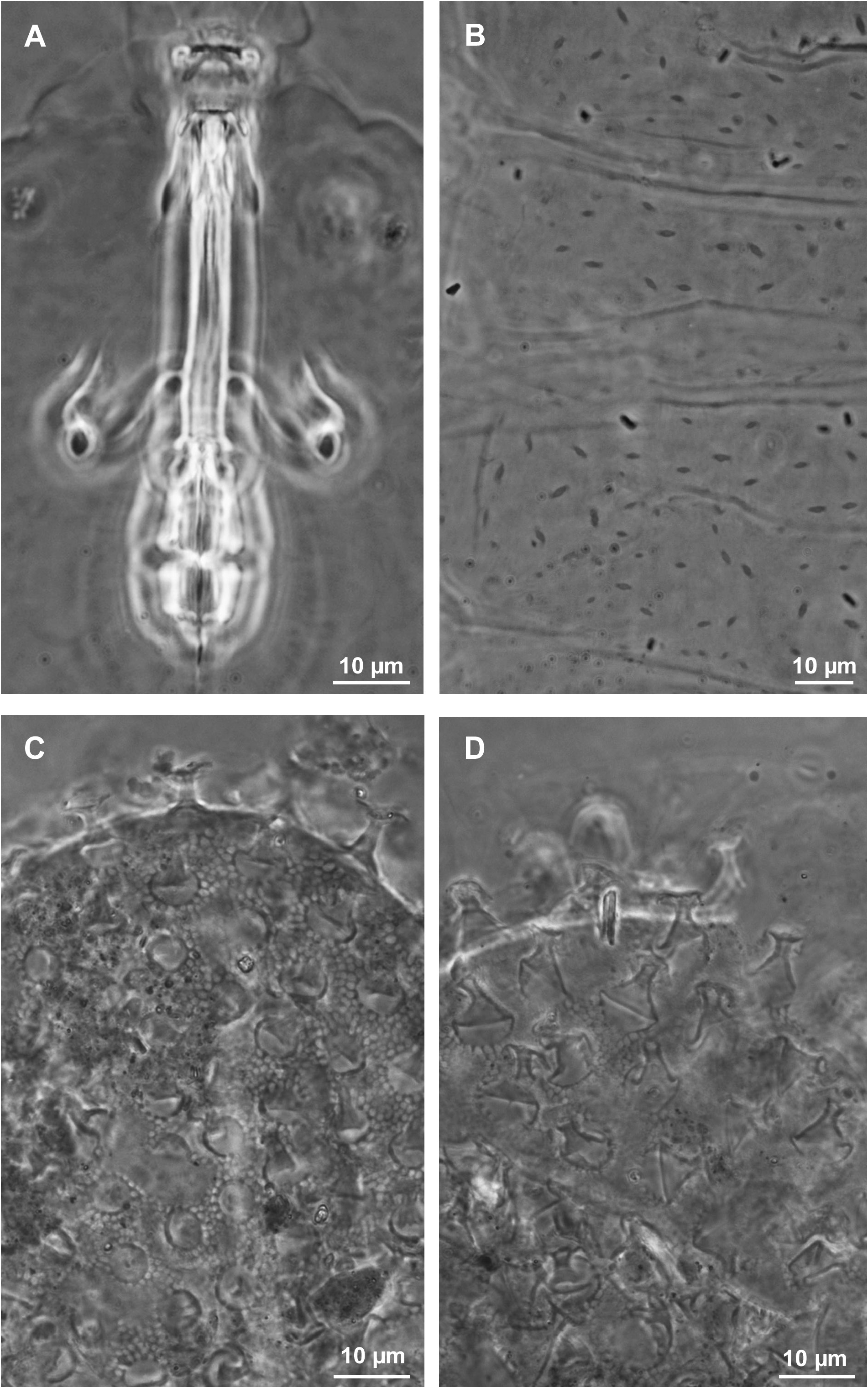
Eggs are laid freely, and are white, spherical or slightly oval. One egg containing a fully developed embryo showed the shape of the buccal-pharyngeal apparatus ( Fig. 4A View Fig ). Processes of the eggshell are in the shape of inverted goblets ( Fig. 4B View Fig ) with conical trunks and well-defined distal discs as large as the process bases (for measurements see Table 4 View Table 4 ). Distal discs concave, with a median small protuberance and, using PhC, with border often smooth, or sometimes slightly jagged, or slightly ragged ( Fig. 4C View Fig ),
but never clearly jagged, serrated or dentate. Surface among processes of the hufelandi type (sensu Kaczmarek & Michalczyk 2017a), i.e., covered by a very thin grid ( Fig. 4D View Fig ). Meshes around the process bases slightly larger and with slightly thicker wires compared with interbasal meshes. Mesh diameter around 0.5 µm.
Comparisons
Macrobiotus azzunae sp. nov. has eggs with processes as inverted goblets and a reticulate eggshell between the processes. Consequently, a comparison must be done with the Macrobiotus species listed by Kaczmarek & Michalczyk (2017a) with hufelandi type eggshells, excluding the species with processes that are not like inverted goblets, and adding the species with hufelandi type chorion eggs described after that publication. The species with hufelandi type chorion eggs that do not have processes as inverted goblets are Macrobiotus acadianus (Meyer & Domingue, 2011) , M. dariae Pilato & Bertolani, 2004 , M. lissostomus Durante Pasa & Maucci, 1979 , M. santoroi Pilato & D’Urso, 1976 , and M. scoticus Stec, Morek, Gąsiorek, Blagden & Michalczyk, 2017 . Moreover, M. azzunae sp. nov. has egg processes with distal discs with a smooth or slightly jagged border, therefore it differs from all the species that have clearly indented, serrated or clearly jagged distal discs, such as: Macrobiotus canaricus Stec, Krzywański & Michalczyk, 2018 , M. crustulus Stec, Dudziak & Michalczyk, 2020 , M. hannae Nowak & Stec, 2018 (whose egg surface is more cribriform than reticulate), M. hibiscus de Barros, 1942 , M. horningi Kaczmarek & Michalczyk, 2017b (which also has very high processes), M. hufelandi C.A.S. Schultze, 1834 , M. humilis Binda & Pilato, 2001 , M. iharosi Pilato, Binda & Catanzaro 1991 , M. joannae Pilato & Binda, 1983 , M. julianae (Meyer, 2012) , M. kamilae Coughlan & Stec, 2019 , M. modestus Pilato & Lisi, 2009 , M. noonragi s Coughlan & Stec, 2019, M. papei Stec, Kristensen & Michalczyk, 2018 (with particularly long filaments starting from the distal disc), M. paulinae Stec, Smolak, Kaczmarek & Michalczyk, 2015 , M. polypiformis Roszkowska, Ostrowska, Stec, Janko & Kaczmarek, 2017 (even with cog-teeth extended to form a long, thin, hair-like and flexible filament), M. punctillus Pilato, Binda & Azzaro, 1990 , M. sapiens Binda & Pilato, 1984 , M. sottilei Pilato, Kiosya, Lisi & Sabella, 2012 .
For the shape of the egg Macrobiotus azzunae sp. nov. differs from M. rawsoni Horning, Schuster & Grigarick, 1978 because this species has only one strip of meshes around each egg process (see Kaczmarek & Michalczyk 2017b); it differs from M. serratus Bertolani, Guidi & Rebecchi, 1996 because in this species the egg surface is porous more than reticulated, with pores small and spaced from each other, and its egg processes have a large, often square, distal disc; it differs from M. seychellensis Biserov, 1994 because the distal disc of the egg processes of this species, even though not dentate, has long and very developed lobes.
The remaining nine species of the hufelandi group should be compared singularly.
Macrobiotus almadai Fontoura, Pilato & Lisi, 2008
Macrobiotus azzunae sp. nov. differs from M. almadai in having a posterior band of teeth in the buccal cavity visible with LM (not visible in M. almadai ), and distal disc with a jagged margin instead of very small teeth as in M. almadai .
Macrobiotus canaricus Stec, Krzywański & Michalczyk, 2018
With LM the margin of the distal disc of M. azzunae sp. nov., never dentate in this species, looks similar to that of M. canaricus , but the SEM images of the eggs of the latter species evidence the presence of an almost dentate disc. Moreover, the peribasal meshes of the eggshell are larger than interbasal ones in the new species while they do not differ from the interbasal ones in M. canaricus ; regarding the animals there are differences in the buccal armature: in M. azzunae sp. nov. the posterior band of teeth is visible with LM (even though poorly) and the three dorsal crests are distinct transverse ridges, while in M. canaricus the posterior band of teeth is visible only with SEM and with LM the dorsal teeth form a transversal ridge weakly divided into three teeth.
Macrobiotus madegassus Maucci, 1993
The new species differs from M. madegassus by the presence of the eye spots (absent in M. madegassus ), pores on the cuticle (absent in M. madegassus ), presence in the buccal armature of posterior band of teeth, even though weak (fully absent in M. madegassus ), buccal tube much larger ( pt of the holotypes 15.9 vs 7), insertion of the stylet supports on the buccal tube much more posterior ( pt of the holotypes 76.1 vs 68), first and second macroplacoid longer ( pt of the holotypes 25.5 and 18.1 vs 21.3 and 12.0), lunules on the hind legs without kerning (crenate in M. madegassus ), eggshell processes with distal disc as large as the base (similar range 3.2–5.2 for both measurements) with respect to that of M. madegassus (disc vs base: 4.3–5.4 vs 2.3–2.6).
Macrobiotus martini Bartels, Pilato, Lisi & Nelson, 2009
The cuticular pores in M. azzunae sp. nov. are much smaller than those of M. martini ; the distal disc of the egg processes in M. azzunae sp. nov. has a diameter similar to that of the process base, while in M. martini the distal disc is much narrower than the base.
Macrobiotus nebrodensis Pilato, Sabella, D’Urso & Lisi, 2017
Macrobiotus azzunae sp. nov. differs from M. nebrodensis by the absence of the cuticular bar near the lunules on the first three pairs of legs (a faint bar is present in M. nebrodensis ). The egg processes of M. azzuane sp. nov. are in higher number on the circumference (29–33) with respect to those of M. nebrodensis (18). In the latter species there are some egg processes very high (up to 20.6 µm), while in the new species process height and shape are more uniform. The difference in the eggshell between meshes around the process base and the others is much less evident in M. azzunae sp. nov. than in M. nebrodensis .
Macrobiotus personatus Biserov, 1990
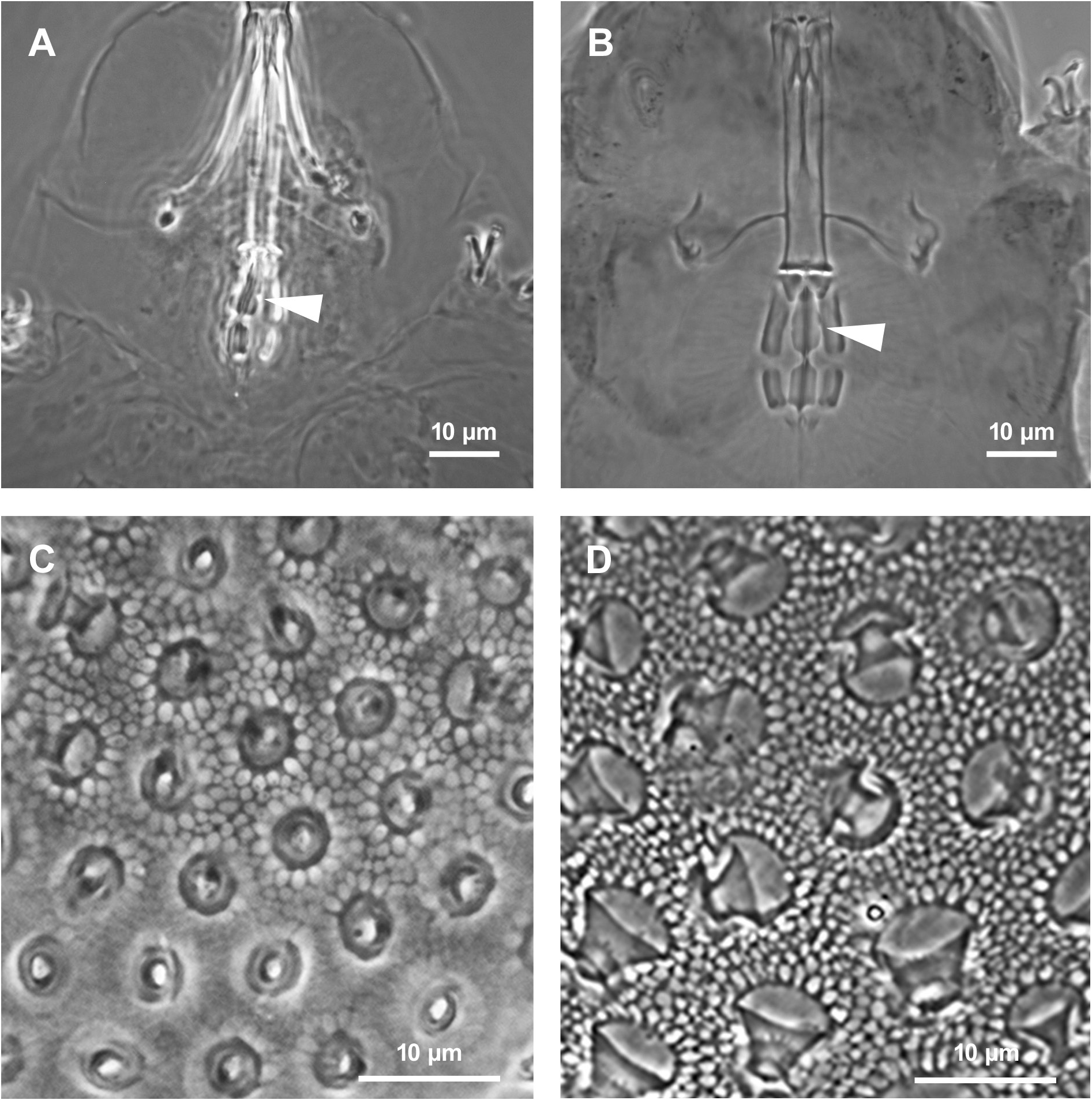
The new species differs from M. personatus by the posterior band of the buccal armature less evident, the presence of a clear constriction in the first macroplacoid ( Fig. 5A View Fig ), in the paratype of M. personatus examined by us barely identifiable ( Fig. 6A View Fig ) and, according to Biserov (1990) usually absent in the type material of that species. The pores on the cuticle of M. azzunae sp. nov. are small, approximately 1 µm in diameter, while in M. personatus they are strongly elliptic and about 3 µm in length ( Fig. 6B View Fig ). Lunules on leg IV are always smooth in M. azzunae sp. nov., sometimes indented in M personatus . With respect to the eggs of M. personatus ( Fig. 6C–D View Fig ), the egg processes of M. azzunae sp. nov. ( Figs 4C–D View Fig , 5C View Fig ) are clearly shorter, 5.4 ± 0.6 vs 9.5 ± 0.5 (range 4.2–6.4 vs 9–10.5) and with a narrower base and distal disc (both 3.2–5.2 vs 7–10.5 and 7–9 respectively). Males are present in the new species, while in M. personatus only females were found ( Biserov 1990), suggesting parthenogenesis in that species.
Macrobiotus sandrae Bertolani & Rebecchi, 1993
The new species differs from M. sandrae for the eggshell shape, with thinner wires of the reticulum and meshes around the processes larger than the inter-process meshes in M. azzunae sp. nov. ( Fig. 5C View Fig ), all meshes similar in size in M. sandrae ( Fig. 5D View Fig ). Figure 5C–D View Fig also show a difference in the process base diameter, narrower in M. azzunae sp. nov. With regard to the animals, M. azzunae sp. nov. differs from M. sandrae by a constriction of the first macroplacoid much more pronounced ( Fig. 5A View Fig ; it is hardly visible in M. sandrae ; Fig. 5B View Fig ). Moreover, in animals of similar size the posterior band of the buccal armature is just less evident in the new species, and lunules on the hind legs are without hint of teeth (but teeth, present in the holotype of M. sandrae , are often difficult to identify in other specimens of that species).
Macrobiotus terminalis Bertolani & Rebecchi, 1993
Macrobiotus azzunae sp. nov. differs from M. terminalis for the absence of granulation on the cuticle (noted only in the redescription of M. terminalis ; see Cesari et al. 2011), for the absence of teeth on the lunules, especially evident on the hind legs of M. terminalis , and for the presence of males, absent in M. terminalis (see redescription by Cesari et al. 2011).
Macrobiotus vladimiri Bertolani, Biserov, Rebecchi & Cesari, 2011
With respect to M. vladimiri , animals of M. azzunae sp. nov. reach a shorter length (up to 410.3 µm vs 515.1 µm), in M. azzunae sp. nov. the posterior band of teeth of the buccal armature is less evident and the lunules on the hind legs are not indented. In M. azzunae sp. nov. the egg diameter without processes ( 64.7–80.6 µm) is less than that of the eggs of M. vladimiri (89.9–92.0 µm); the processes are shorter ( 4.2–6.4 µm in the new species vs 6.5–8 µm in M. vladimiri ). In the new species the base process diameter is narrower ( 3.2–5.2 µm) than in M. vladimiri ( 5.1–7.3 µm), the distal disc is weakly or not jagged (clearly jagged in M. terminalis ). In M. azzunae sp. nov. males are present, while they are absent in M. vladimiri .
Genetic distances
The ranges of uncorrected genetic p-distances between M. azzunae sp. nov. and the other species of the M. hufelandi group (Supp. file 7, Supp. file 8, Supp. file 9, Supp. file 10), are as follows:
18S 0.1–5.6%, with the most similar being M. sandrae from Germany (present paper)
28S 0.1%, with the only available M. vladimiri from Spain ( FJ435751 View Materials –5)
ITS-2 7.7–32.2%, with the most similar being Macrobiotus vladimiri ( MN888347 View Materials ) from Finland
COI 6.3–25.6%, with the most similar being Macrobiotus sandrae ( HQ876574 View Materials , HQ876577 View Materials , HQ876578 View Materials , HQ876579 View Materials , HQ876581 View Materials ) from Germany
The COI dataset is the most complete and informative for species delimitation investigation. Both phylogenetic reconstructions on the COI dataset resulted in the same topology, and thus the ML tree was utilized for the PTP analysis ( Fig. 7 View Fig , left), which shows 14 putative species clusters: M. crustulus , M.hannae , M. cf. recens , M.canaricus , M.hufelandi , M. cf. hufelandi sp.1, M. terminalis , M. cf. terminalis , M. wandae , M. macrocalix , M. cf. macrocalix , M. vladimiri , M. sandrae and M. azzunae sp. nov. This subdivision is further validated by both the ABGD and the haplotype network analysis ( Fig. 7 View Fig , centre and right). Present molecular data therefore confirms the validity of the erection of M. azzunae sp. nov.
Table 3. Measurements (in μm) and pt of selected morphological structures of individuals ofMacrobiotus azzunae sp. nov. mounted in Faure-Berlese. Abbreviations: Nr = number of specimens/structures measured; Range = smallest–largest value; SD = standard deviation. * Three animals below 200 μm in length were not considered in the analysis. Two were not measurable, while data of the only measurable animal below 200 μm is available in the supplementary material (Supp. file 1).
| Character | Nr | Range µm | Mean ± SD | Holotype µm | |
|---|---|---|---|---|---|
| Body length | 10 * | 246.0–410.3 | 374.0 | ||
| Buccal tube length | 13 | 29.4–38.5 | 36.6 | ||
| pt | µm | pt | |||
| Buccal tube external width | 13 | 11.6–15.9 | 13.3 ± 1.2 | 5.8 | 15.9 |
| Stylet support insertion point | 13 | 75.1–80.5 | 76.7 ± 1.5 | 27.9 | 76.1 |
| Placoid row | 13 | 50.1–57.7 | 51.8 ± 2.4 | 18.9 | 51.5 |
| Macroplacoid row | 13 | 37.7–48.7 | 44.6 ± 3.1 | 15.7 | 42.7 |
| First macroplacoid | 13 | 24.3–29.7 | 26.6 ± 2.2 | 9.3 | 25.5 |
| Second macroplacoid | 13 | 16.0–20.7 | 18.3 ± 1.5 | 6.6 | 18.1 |
| Microplacoid | 13 | 5.9–8.6 | 6.9 ± 1.0 | 2.6 | 7.0 |
| External Claws III main branch | 13 | 27.1–31.3 | 29.8 ± 1.3 | 11.5 | 31.3 |
| External Claws III secondary branch | 13 | 20.1–25.8 | 22.5 ± 1.6 | 8.1 | 22.1 |
| Internal Claws III main branch | 13 | 24.9–30.2 | 27.7 ± 1.6 | 10.2 | 27.9 |
| Internal Claws III secondary branch | 13 | 18.8–24.2 | 21.4 ± 1.7 | 7.2 | 19.7 |
| Posterior Claws IV main branch | 9 | 29.1–34.6 | 32.7 ± 1.8 | 12.2 | 33.2 |
| Posterior Claws IV secondary branch | 9 | 21.6–24.9 | 23.8 ± 1.6 | 9.1 | 24.9 |
| Anterior Claws IV main branch | 10 | 28.2–32.5 | 30.1 ± 1.9 | 11.1 | 30.3 |
| Anterior Claws IV secondary branch | 10 | 20.4–26.2 | 23.2 ± 1. 7 | 8.8 | 24.0 |
Table 4. Measurements (in μm) of selected morphological structures of the eggs of Macrobiotus azzunae sp. nov. mounted in Faure-Berlese. Abbreviations: Nr = number of eggs/structures measured; Range = smallest–largest value; SD = standard deviation.
| Character | Nr | Range | Mean ± SD |
|---|---|---|---|
| Egg diameter without processes | 2 | 64.7–80.6 | – |
| Egg diameter with processes | 2 | 72.4–89.2 | – |
| Process nr on egg circumference | 2 | 29–33 | – |
| Process height | 10 | 4.2–6.4 | 5.4 ± 0.6 |
| Process base width | 10 | 3.2–5.2 | 4.2 ± 0.5 |
| Distal disc width | 10 | 3.2–5.2 | 4.4 ± 0.6 |
| Inter process distance | 10 | 2.7–4.8 | 3.5 ± 0.5 |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |