Toxicodryas blandingii (Hallowell, 1844)
|
publication ID |
https://doi.org/10.11646/zootaxa.4965.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:67F8DED5-EBDB-46F8-9EAE-88B37446C9FA |
|
DOI |
https://doi.org/10.5281/zenodo.4741193 |
|
persistent identifier |
https://treatment.plazi.org/id/153987C4-E22D-B657-D9D2-54001679FC47 |
|
treatment provided by |
Plazi |
|
scientific name |
Toxicodryas blandingii |
| status |
|
Toxicodryas blandingii (Hallowell, “1844” 1845)
( Table 1, Figs. 5–8 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
Dipsas Blandingii : Hallowell (“1844” 1845:170); type locality: “ Liberia, West Africa. ”
Triglyphodon fuscum: Duméril, Bibron & Duméril (1854:1101) View in CoL ; type locality: “ Grand-Bassam , sur la Côte d’Ivoire (Guinée)” [ Ivory Coast].
Dipsas fasciata: Fischer (1856:84) ; type locality: “ Peki ( West-Afrika)” [ Ghana].
Dipsas valida: Fischer (1856:87) ; type locality: “ Edina ( Grand Bassa County, West-Afrika)” [ Liberia].
Dipsas globiceps: Fischer (1856:89) ; type locality: “ Edina ( Grand Bassa County, Liberia, West-Afrika).”
Toxicodryas Blandingii: Hallowell (1857:60); comb. nov.
Dipsas Fischeri: Jan in Duméril (1859:212); no type locality provided.
Triglyphodon fuscum var. obscurum: Duméril (1861:211) View in CoL ; type locality: “ Côte d’Or ” [ Ghana].
Dipsas regalis: Jan (1871:3, Livraison 38, pl. vi, fig. 2) in Jan & Sordelli (1870 –1881); type locality: “ Côte d’Or ” [ Ghana].
Dipsas globiceps var. tumboensis: Müller (1885:688) ; type locality: “ Tumbo-Insel ” [ Guinea].
Boiga blandingi occidentalis: Stucki-Stirn (1979:377) ; inferred type locality: “Besongabang” Cameroon.
Boiga blandingi subfulva: Stucki-Stirn (1979:381) ; type locality: none provided, but limited to Cameroon according to book title .
Toxicodryas blandingii was originally described by Hallowell (1845:170) based on a single specimen collected by his friend, “Dr. Blanding,” in Liberia. The dorsum and venter of the specimen was noted to have a “light yellow” color with a series of blotches of “leaden colour.” This specimen reportedly possessed 2 preoculars, 2 postoculars, 272 ventrals, 131 subcaudals, body length (i.e., SVL) of 1.22 meters, and tail length of 0.39 meters. Hallowell (1854) provided additional details of the specimen’s teeth, noted it had 17 “rows of scales,” and corrected the tail length to 0.37 meters. Hughes & Barry (1969), Wallach et al. (2014) and Uetz et al. (2019) stated that the type was lost, which is consistent with Malnate (1971), who did not list a type specimen from the ANSP collection. Wallach et al. (2014) noted the type was a 1.67 m specimen, slightly longer than the total length of 1.61 m reported by Hallowell (1845) in the original description, but the longer measurement is likely a typographical error in reference to the latter citation (V. Wallach, pers. comm.). A query by EG to the Philadelphia Academy of Sciences in spring 2020 resulted in location of the type specimen (ANSP 10083, Fig. 6 View FIGURE 6 ), and a redescription of this specimen is provided below.
According to Loveridge (1957:269), the name Dipsas Fischeri was proposed by Jan (in Duméril 1859) to combine the minor color pattern variants Dipsas fasciata , D. valida , and D. globiceps named by Fischer (1856). Pel (1852:171) coined the name Dipsas regalis , and as translated by Savage & McDiarmid (2017:73), Pel stated, “the third species of venomous snake, Naja atropos , belongs to cobras (spectacled snakes) and reaches a length of 6 to 7 feet [ 1.8–2.1 m]. Its color is entirely black... As this snake in general shows much similarity to a tree snake, Dipsas regalis , which equals it in color and size, but is not venomous.” Perhaps because of this poor description, Boulenger (1896:78) attributed the latter name to Jan & Sordelli (1870 –1881), who provided an illustration that served as an appropriate description. Jan listed the name in the Index des Planches for Livraison 38 as Dipsas cynodon Cuv. variété? ( D. regalis Schlegel ), but according to Savage & McDiarmid (2017:73), the attribution to Hermann Schlegel is in error because he never used the name in any publication. Müller (1885:687) seemed to suggest that Jan illustrated his specimen (collected by Dr. Mähly from “Goldküste” [i.e., Gold Coast or modern-day Ghana]) from Basel, but Hughes & Barry (1969:1020) listed a personal communication from M.S. Hoogmoed, who noted the type of D. regalis (specimen “Leiden 958”) was collected by Pel in February 1844 from Accra, Ghana.
Hallowell (1857:60) coined the genus Toxicodryas because he noticed that his specimen of T. blandingii had a “single channelled posterior tooth on each side... and therefore...[it] cannot belong to the genus Triglophodon [sic] of Dum. and Bibron, which has three.” Subsequent herpetological publications in the 19 th and early 20 th centuries seemingly ignored Hallowell’s new genus and continued to recognize the taxon in either the genus Dipsas (e.g., Mocquard 1896) or more commonly, Dipsadomorphus (e.g., Boulenger 1896, 1919; de Witte 1933). Schmidt (1923) transferred the taxon to the genus Boiga in his opus on Congolese snakes, recognizing B. ( Toxicodryas) blandingii and B. ( Toxicodryas) pulverulenta , an action that was followed by most subsequent authors for decades. Based on the placement of African Boiga in the “ Dipsadidae : Lycodontinae ” by Underwood (1967), Welch (1982) seems to have been the first to recognize the genus Toxicodryas for both species of the genus, an action followed by Meirte (1992) and observed by most herpetologists in the 21 st century (e.g., Uetz et al. 2020).
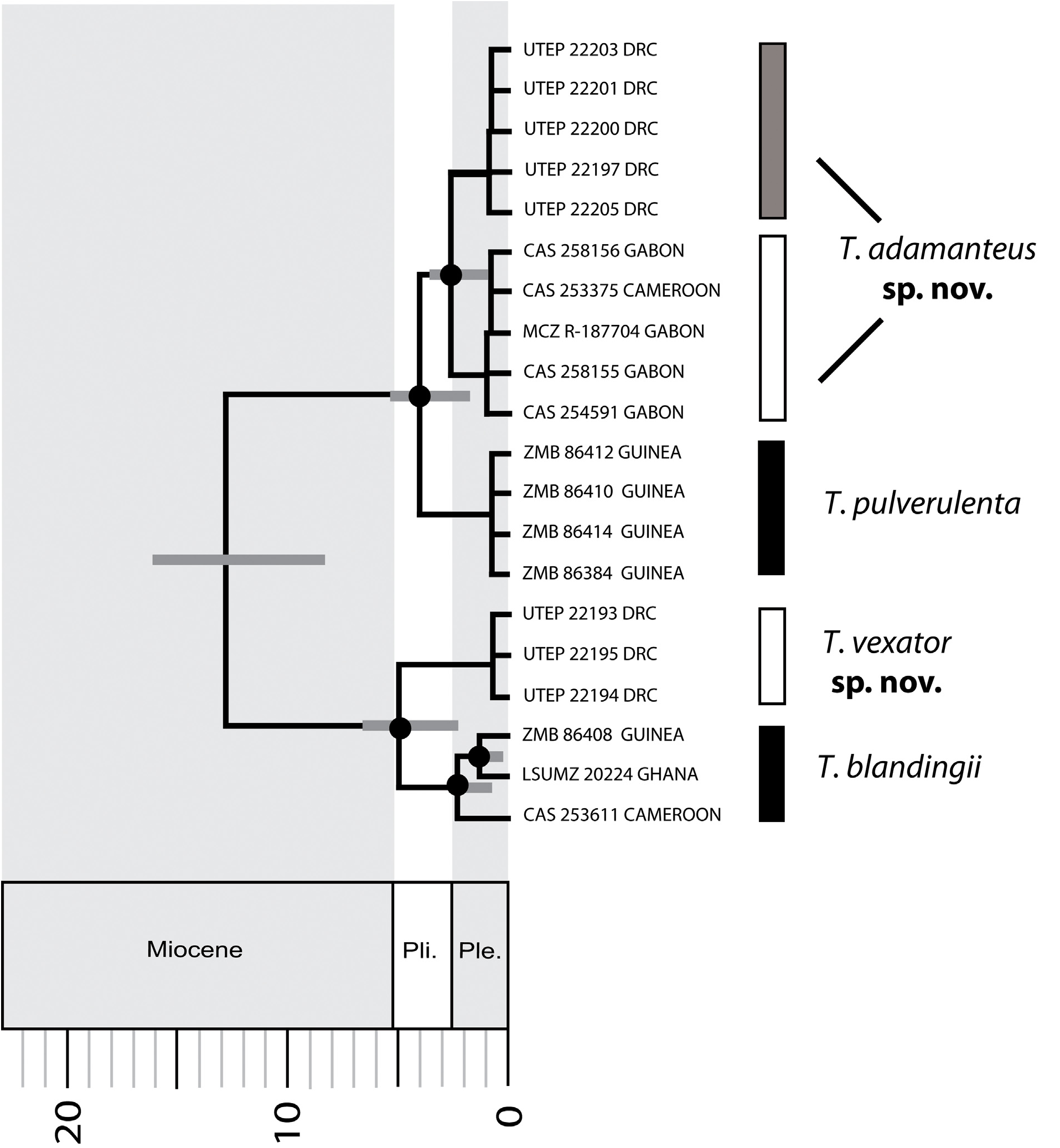
Boulenger (1896) included all of the above West African, 19 th- century names in the synonymy of Dipsas ( Toxicodryas) blandingii . Because the dubious subspecies described by Stucki-Stirn (1979) both occur in Cameroon, where one molecular sample (CAS 253611) from Allen et al. (in press) is recovered in a well-supported clade with West African samples ( Fig. 2 View FIGURE 2 ), we confirm the taxonomic nomenclature of Wallach et al. (2014) in treating these taxa as synonyms of T. blandingii . Marques et al. (2018) noted that northwestern Angolan records from “Chinchoxo,” “Piri-Dembos,” and “Quirimbo” by Peters (1877), Bocage (1895), Parker (1936), and Hellmich (1957a, b) are attributable to T. pulverulenta . However, the morphometric data provided by Hellmich (1957b) for Angolan snakes are inconsistent with the size and scale rows of the latter species, and herein, we consider his records to be attributable to T. vexator sp. nov.
Diagnosis. Toxicodryas blandingii , as recognized herein, is restricted to West Africa and west-central Africa (west of the confluence of the Congo and Ubangi rivers), defined by the following combination of characters: maximum SVL> 1 meter (vs. maximum SVL < 1 meter in T. pulverulenta and T. adamanteus sp. nov.); DSRN 23–25 (vs. 19–21 in T. pulverulenta and 18–23 in T. adamanteus sp. nov.); DSRM 21–25 (vs. 19–21 in T. pulverulenta and 18–21 in T. adamanteus sp. nov.); cloacal plate usually divided (vs. divided or undivided in T. vexator sp. nov., and always undivided in T. pulverulenta and T. adamanteus sp. nov.); adult males glossy or velvety black with a yellow venter, and adult females light brown, gray, or yellowish-brown with light-brown or cream cross-bars on the flanks, with yellowish-brown venters (vs. both sexes brown to pink with darker cross-bars that often enclose a whitish spot, and the dorsum and venter sprinkled with fine dark brown or black spots in T. pulverulenta and T. adamanteus sp. nov.); hemipenis relatively short and massive (i.e., broad), proximal third covered with spines, distal two-thirds dimpled with a flattened apex (vs. relatively long with long spines mid-way along the shaft that decrease in size towards the apex and base, and with a domed apex in T. pulverulenta and T. adamanteus sp. nov.); venom toxicity LD 50 = 2.85–3.55 mg /kg in mice (vs. venom toxicity LD 50 = 4.88 mg /kg in mice for T. vexator sp. nov.).
Redescription of the holotype. ANSP 10083 ( Fig. 6 View FIGURE 6 ) adult female in poor condition, 1330 mm SVL; head triangular and distinct from neck, 1.92% of SVL ( 25.5 mm); right loreal missing, left loreal partially obscured by supralabials due to cranial damage, upper side tapering superiorly; body triangular; tail moderately long ( 400 mm; 30.1% of SVL). Supralabials –/9, –/4 th, 5 th, and 6 th contacting orbit; infralabials 14/13, 1 st on each side in contact behind mental, 1 st –4 th /1 st –4 th contacting anterior chin shields and 4 th –7 th /4 th –7 th contacting posterior chin shields; 2 preoculars; 3 postoculars (on left, missing on right); temporals –/2 + 2; 2 internasals; nasal divided (on left, missing on right); frontal slightly longer than wide, only left side undamaged; dorsal scale rows 23 one head length posterior to jaw rictus, 23 at midbody, 17 one head length anterior to vent, smooth and oblique, vertebral scales broad and apically flattened; ventrals 273 (standard), 271 (Dowling); cloacal plate undivided; paired subcaudals 132.
Coloration (in preservative). After approximately 176 years in preservation, specimen is faded, with creamy tan background color in dorsal and ventral views. Brown markings on posterior edge of supralabials and dorsum of head. Irregular brown and dark brown blotches and saddles on dorsum from neck to tip of tail ( Fig. 6 View FIGURE 6 ).
Variation. Morphometric variation of Toxicodryas blandingii is shown in Table 1. Müller (1885:688) provided data for a snake from Ghana with 15 infralabials and noted that most of its scales have two “Endgruben” [terminal pits], which likely refer to apical pits. In his description of Dipsas globiceps var. tumboensis Müller (1885) noted his specimen from Guinea ( Fig. 7 View FIGURE 7 ) had 147 subcaudals. In snakes from West Africa (without separating by sex), Angel (1933) noted a temporal formula of 2 + 2 or 2 + 3, 21–25 scales at midbody, 240–289 ventrals, 120–147 subcaudals, either an undivided or divided cloacal plate, and a maximum total size of 2290 mm, and nearly verbatim variation was listed by Villiers (1950a), Doucet (1963), Stucki-Stirn (1979), Chippaux (2006), and Trape & Mané (2006). However, in snakes from Ghana, Swiecicki (1965:302) noted a maximum total length of 2450 mm for a “black form” individual, Gauduin (1970) listed the maximum total length as 2700 mm ( 600 mm tail) for Cameroon, Chirio & LeBreton (2007) provided a slightly larger total length of 2740 mm for Cameroon, and Luiselli et al. (1998a) noted a maximum size of 2800 mm, presumably for Nigeria. Villiers (1951) noted an unsexed individual from Benin with 115 subcaudals. Cansdale (1965) documented 21–25 scales at midbody, and Segniagbeto et al. (2011) documented snakes from Togo with 19–24 scales at midbody and 102–159 subcaudals.
In our examined specimens, temporal formula includes the variation noted by Angel (1933), but is more extensive (2 +5, 3 + 4, 3 + 3, 1 + 5, 3 + 2, or 2 + 4), and either supralabials 3–5 or 4–6 contact the eye, which is consistent with the observations of Angel (1933), Villiers (1950b), and Chippaux (2006); the latter author also noted that sometimes only 2 supralabials contact the eye. Rasmussen (1997a) noted specimens with the 4th–5th, 5th–7 th, or 4th–7 th supralabials in contact with the eye, and in general, this species has sloping and smooth scales with apical pits, and the vertebral row is greatly enlarged. The holotype, one male and one female from Liberia ( Loveridge 1941; Johnsen 1962), one male from Gabon ( Pauwels et al. 2002b), one male from DRC (RBINS 10888), and a juvenile from Cameroon ( Werner 1897) are unusual in having an undivided cloacal plate, because all other examined specimens have a divided cloacal plate, including the type specimens of Dipsas fasciata , D. valida , and D. globiceps ( Fischer 1856) . Rasmussen (1997a) remarked that his specimens have either a divided or undivided cloacal plate. In his book on West and Central African snakes, including countries west of the Congo River, Chippaux (2006:154) noted the anal [cloacal plate] is sometimes entire, but more often divided. Trape & Mané (2006) stated that the cloacal plate is almost always divided. Segniagbeto et al. (2011) noted individuals with divided or undivided cloacal plates in Togo.
Combined descriptions by Fischer (1856) of the teeth of Dipsas fasciata , D. valida , and D. globiceps (all now synonyms of T. blandingii ) suggest the species has 9 maxillary teeth that increase in size posteriorly, followed by two fangs (three on the right side in one specimen), and 12 mandibular teeth, which decrease in size posteriorly. In snakes from Ghana, Leeson (1950) noted 10–11 maxillary teeth, becoming larger posteriorly, and two fangs followed by a shorter fang; fourteen large palatine and pterygoid teeth, and 15 mandibular teeth (anterior ones largest). Taylor & Weyer (1958:1217) described a Liberian specimen with 9–10 maxillary teeth (on different sides) that increase in size from the 1 st to 4 th tooth, and then become subequal; two fangs occur after this series of teeth, and after a short diastema, there is a third fang with only traces of a groove. A second Liberian specimen had 10 maxillary teeth followed by three fangs, the last of which had only “a suggestion of a groove.” Based on specimens ranging from Guinea to Congo, Rasmussen (1997a:98) noted 10 maxillary teeth followed by three enlarged, furrowed venomous teeth, with the 3 rd fang slightly smaller than the previous two.
Fischer (1856) provided detailed descriptions of the color patterns of West African Dipsas fasciata , D. valida , and D. globiceps (all now synonyms of T. blandingii ), which seem to suggest he examined a subadult male that had been kept in alcohol for a long (unspecified amount) time ( D. fasciata ), an adult female ( D. valida ), and a subadult that retained juvenile coloration ( D. globiceps ). In his description of Dipsas globiceps var. tumboensis Müller (1885:689) noted his specimen from Guinea ( Fig. 7 View FIGURE 7 ) had a gray-reddish dorsum with 30 black transverse bands, usually containing milky white spots. The frontal, supraoculars, and occipital scales had large black spots, the labial scales and postoculars were edged with black, and the head shields had multiple milk-white speckles. The tail was bright red with dark, irregular transverse bands. Aspects of this coloration description are highly unusual for this species (e.g., bright red tail), and more typical of T. pulverulenta , but the black edging of the labial scales, number of preoculars (3), supralabials in contact with the eye (5 and 6), ventrals (269), and subcaudals (147) clearly indicate this taxon is a synonym of T. blandingii ( Fig. 7 View FIGURE 7 , Tables 1–2).
Mocquard (1887:80) described a recently collected, unsexed subadult (“la longueur du tronc” [trunk length] 1.1 m) from Gabon as having a dorsal color of a general tint of Burgundy with slightly darker spots on the flanks that have a dirty white spot a little above their lower edge. Mertens (1938) described an adult male from Cameroon as solid black dorsally and ventrally, with the exception of the anterior third of the venter, which was white, but the ventral scales had dark gray edges. The labial scales were gray with vertical black borders. Villiers (1950b) described the color pattern of an unsexed individual from Ivory Coast as sooty black or brownish in places on the dorsum; underside iridescent dark gray posteriorly, becoming whitish anteriorly, with the posterior edge of the ventrals edged with gray; underside of head white, and labials whitish and edged in black. Another unsexed individual from Liberia was described as bluish black above, yellow below; supralabials yellow with black edges, and posterior part of venter and underside of tail black. A third unsexed individual from Liberia had identical coloration to the latter one, except for the presence of whitish bars on the neck. Leeson (1950) noted that snakes from Ghana have a dull green or gray dorsum. Monard (1951:162) described an unsexed individual from Cameroon as a beautiful light redbrown, “barré” [barred] with dark brown. Taylor & Weyer (1958:1217) described a Liberian brown-phase female with pale grayish green on the ventral side of the head and neck, merging into gray with “a greenish cast” 12.7 cm posteriorly, and at 40.6 cm behind the head, it transitioned into plain tan to the tip of the tail. Isemonger (1962:12) remarked that this species has a “delicate bloom on the skin.” Cansdale (1965:43) described a highly unusual color pattern for juveniles by noting that “the young brown form is pink with irregular chocolate markings that break up its outline very effectively and make it difficult to pick out in a tree or shrub.” Leston & Hughes (1968:753) described an unusual specimen from Ghana as “pale grey with darker greyish-green transverse bands, the bands irregular but more or less diamond shaped on each side. The ventrals are also grey but more glossy.”
Groves (1973:107) described the coloration of hatchlings from a captive Liberian female as “light grey background colour with pinkish undertones; black, roughly oval, lateral blotches narrowing as they approach the midline, where many of them fail to conjoin; top of head light grey; belly dark grey.” Rasmussen (1997a:98) noted the scales of his specimens were dull and almost dusty, a sentiment also shared by Cansdale (1965). Adult males were solid black on the dorsum and yellow on the venter (becoming black posteriorly), whereas adult females were noted to be gray, brown or yellow-brown on the dorsum and yellow-brown on the venter, sometimes without transverse bands. Hughes (2000:8) noted juvenile and subadult (approximately 1 meter in total length or less) snakes had a dorsal coloration that was “a distinctively bright and contrasting pattern of chocolate brown blotches.” He noted that most male specimens lose this coloration as they age, becoming increasingly melanistic, and although exceptions are possible, this melanistic progression does not seem to occur in females.
Chippaux (2006:154) noted there are two dorsal color morphs: (1) uniform black or dark blue with “reflets veloutés” [velvety reflections] or (2) gray with darker, poorly defined transverse spots. The venter was noted as dull yellow to charcoal gray, and juvenile coloration as light brown with darker transverse ring-shaped spots. Stucki-Stirn (1979), perhaps confused by the two color morphs ( Lawson 1993; Rasmussen 1997a), described Boiga blandingi occidentalis for Cameroonian specimens that were uniform black or bluish black, whereas B. blandingi subfulva was named for Cameroonian specimens that were yellowish brown with faint whitish or dark brown diamondshaped blotches. Lawson (1993) attributed these color morphs to sexual dimorphism, but Hughes (2000) suggested exceptions are possible. Trape & Mané (2006:168) added, although there are a few exceptions, the differences in coloration are clearly associated with the sex and age of the specimens. Greenbaum & Carr (2005:15) documented the color in life of an adult female from Guinea as “dorsum and flanks were pinkish tan with 39 brown blotches outlined in a creamy tan border along the flanks. The dorsum of the head is brown; the labials are tan with brown outlines, and the venter is white.” Pauwels et al. (2020) documented an adult specimen from Gabon that was uniformly beige in life. Based on photos of a subadult of unknown sex from Mbiliki, Cameroon ( Fig. 5G View FIGURE 5 ) and a juvenile of unknown sex from Gamba, Gabon ( Fig. 5H View FIGURE 5 ), the base of the tongue is bluish black, and the forked tip is silvery gray.
Hemipenis. Doucet (1963:299) illustrated ( Fig. 8 View FIGURE 8 ) and described the hemipenis of a specimen from Ivory Coast as non-bifid, short and massive [i.e., broad], and flattened. Proximal third covered with spines except at the level of a “mamelon” [nipple] near the root. The distal two-thirds are dimpled, and the apex is flattened. Hemipenes of our examined specimens of T. blandingii had a simple, subcylindrical shape, simple sulcus spermaticus, and spinose ornamentation with a rough apical structure.
Diet. In his description of Dipsas globiceps var. tumboensis Müller (1885) noted his specimen from Guinea had a bird’s egg in its stomach. Mocquard (1896) mentioned this species eats lizards in Guinea. Sternfeld (1909) suggested that small birds are the main food of this species in Cameroon, and this contention was repeated by Hughes (2000). Villiers (1950a) noted both species of Toxicodryas in West Africa feed mainly on birds. Villiers (1950b) remarked that one of the listed specimens (either from Ivory Coast or Liberia) had a bird in its stomach. Monard (1951) noted a snake from Cameroon had an Orange Weaver ( Ploceus aurantius ) in its stomach. Villiers (1951) documented a bird in the stomach of a snake from Benin, and an Agama sp. ( sensu Leaché et al. 2017) in the stomach of a snake from Togo. Cansdale (1955:31) suggested that “it is reputed to be an egg-eater in the Gold Coast [ Ghana] and has the same Twi name as the true Egg-eating Snake; but I ... cannot confirm it from the literature.” Cansdale (1965) repeated the latter remark and added that they will eat eggs in captivity.
Dekeyser (1955) cited Villiers (1955), who supposedly noted a large number of bats in a West African T. blandingii , and the identity of these bats was later mistakenly attributed to Lavia frons by Wickler & Uhrig (1969) and Happold & Happold (2013), perhaps because this bat species was mentioned earlier in the same paragraph by Dekeyser (1955). However, Villiers (1955) is a study about parasites in African vertebrates, and there is no mention of Toxicodryas or any bat species. It is likely that Dekeyser (1955) was referring to Villiers (1956), who documented a snake from Guinea that was found in the cracks of a cave’s ceiling, and its stomach contained several bats in the genus Hipposideros .
Hughes (2000) reported complaints by local people in Ghana that this species will raid eggs from domesticated poultry, but it is possible that the true culprit was Naja guineensis ( sensu Wüster et al. 2018) , which is also long and has a tendency to be melanistic in adults. Pitman (1958:84) quoted T.S. Jones from Sierra Leone who shot three snakes as they raided Village Weaver nests as “the snakes were pushing their heads into one nest after the other and taking the young ones, numbers of which were found in each.” Another individual “almost certainly of this species” was seen by Jones preying upon young birds of the Little African Swift ( Apus affinis abessynicus ). Taylor & Weyer (1958:1217) documented a snake from Liberia that was found electrocuted with another “large black snake” that the local people claimed had been chasing it. The stomach contents contained one subadult and two nestlings of Spermestes cucullata . Woodward (1960) noted the species eats lizards, mice, small bush rats, frogs, and bats in Nigeria, and similar findings were noted by Menzies (1961) for Sierra Leone, Cozens (1961) for Nigeria, and Pitman (1962) for Nigeria. Based on a photograph of an adult black-phase snake shown in Woodward (1960:173), Barry (1961), Dunger (1961), Menzies (1961), and Greene (1989) corrected the identification from T. pulverulenta to T. blandingii . Dunger (1961) noted that his “dead specimens” from Nigeria contained fledgling birds and immature rats.
Jones (1961) documented a 1.8-meter snake from Sierra Leone that contained the remains of one Agama sp. lizard and eight bats, which were identified as Neoromicia tenuipinnis , Tadarida sp. , and Mops condylurus . A second Sierra Leonean snake, which was killed as it emerged from a roof, also contained Tadarida sp. bats. Happold & Happold (2013) cited Jones (1961) to document T. blandingii as a predator of Nycteris hispida , possibly in error, because none of the taxa noted by the latter author are synonyms of this species. On another occasion in Sierra Leone, the latter author shot three snakes that were in a tall palm tree devouring nestlings of the Village Weaver ( Ploceus cucullatus ). Johnsen (1962) reported an adult female from Liberia that was preserved with a passerine bird ( Spermestes fringilloides ) in its mouth. Menzies (1966) documented prey items in snakes from Sierra Leone, including agamid lizards, chameleons, and bats. Leston & Hughes (1968) documented snakes from Ghana with stomach contents that included village weavers ( Ploceus cucullatus ), other birds, and Agama sp. lizards ( sensu Leaché et al. 2017). Leston (1970) noted the stomach contents of several snakes from Ghana, including Agama sp. lizards, a fiscal shrike, a subadult weaver bird, and a Bronze Mannakin ( Spermestes cucullata ). Six of seven of these snakes were found in trees, and the 7 th was electrocuted when it climbed onto a power line.
Groves (1973) documented several captive hatchlings that refused to eat lizards and frogs, but mice were accepted, contrasting with patterns documented by Greene (1997) as noted by Hughes (2000). Lawson (1993) described two snakes from Cameroon —an adult had eaten an Agama sp. lizard ( sensu Leaché et al. 2017) and a juvenile contained a small bat. Luiselli et al. (1998a) found that in Nigeria, juvenile snakes (< 800 mm SVL) ate only lizards, including Agama sp. , Trachylepis sp. and Lygodactylus conraui , whereas larger individuals ate Agama sp. lizards, Nectarinia sp. sunbirds, and other birds and rodents (see also Luiselli et al. 1998b).
In a paper on Guinean snakes, Böhme (2000) classified the species as relatively common and nocturnal, and its diet included bird adults, nestlings and eggs, lizards with well-developed limbs, bats, and frogs. Hughes (2000) noted juvenile and subadult snakes (less than 1300 mm SVL) ate skinks, mice, birds, and Agama sp. lizards ( sensu Leaché et al. 2017), whereas adults preyed upon Agama sp. lizards and birds (including bulbuls, doves, and weavers). Pauwels et al. (2002b) noted an adult male Rhampholeon spectrum in the stomach of a juvenile from Gabon. Greenbaum & Carr (2005) found an adult female actively climbing through a tree during the day in Guinea, and stomach contents included three bats ( Mops condylurus ) and the remains of a Chamaeleo lizard. Akani et al. (2007) dissected snakes from oil palm plantations in Nigeria (found as high as 25 meters above ground) and found birds and bats in the stomachs of some individuals. Pauwels et al. (2019a) reported a suspected predation of a grey-necked rockfowl ( Picathartes oreas ) by T. blandingii in a cave in Gabon.
Parasites. Butler & Reid (1990) documented two specimens from Nigeria that contained cestodes in their intestines .
Predators. Pauwels et al. (2017c) reported a Naja melanoleuca that had preyed upon a similar-sized T. blandingii in a house garden in Gabon.
Behavior. Barry (1961:43) mentioned that he had captured a snake in an oil palm in Ghana at 2 P. M. in the afternoon, and shortly thereafter, it regurgitated a “freshly swallowed” bat, suggesting the snake was preying upon roosting bats during the day. Groves (1973:107) noted that, in captivity, an adult female from Liberia was active during the day, whereas an adult male of uncertain origin was mostly nocturnal. The latter author also noted that “a number of authors” have described the constricting behavior of Boiga (as recognized at that time to include Toxicodryas ), but this was never observed in his captive animals. Young (1983) and Greene (1989) confirmed that this species does not constrict its prey, but they can be manipulated and controlled with loops of the snake’s body. The former author noticed that captive snakes prefer to feed in low light or complete darkness, suggesting nocturnal habits. Lawson (1993) described snakes from Cameroon as diurnal and nocturnal. Rasmussen (1997a) listed the species as mainly nocturnal and arboreal, sometimes occurring 20–30 m above ground in trees. Luiselli et al. (1998a) documented numerous individuals during daylight hours (nocturnal collecting was impossible because of security concerns), usually in trees 2–10 meters above the ground, and they found significantly more diurnal snakes in the wet season compared to the dry season. However, Luiselli et al. (1998b:131) described the species as “mostly nocturnal,” which they suggested could reduce competition for prey with the sympatric, “strictly diurnal” and arboreal species Dendroaspis jamesoni . Rödel & Glos (2019) observed a large individual hunting in the canopy of a tree in rainforest, carefully inspecting one branch of the tree after another, above a rocky torrential stream at night in Liberia (MOR, pers. obs.).
Among the 19 snake species recorded within the garden of a villa in Yenzi ( 2.77261° S, 10.03403° E), Gamba, Nyanga Province, southwestern Gabon (forest-savanna mosaic), inhabited by one of the authors from 2004 to 2011 (OSGP, unpubl. data), T. blandingii was, by far, the most commonly encountered species of snake ( Fig. 5H View FIGURE 5 ). The snakes were regularly observed at night hunting for sleeping Agama sp. ( sensu Leaché et al. 2017) on bushes and walls, and birds in their nests. If closely approached, the snakes were often aggressive, and if caught, a number of them, rather than fleeing when released, preferred to confront and bite. However, Pauwels & Vogel (2011:24) documented one individual from Yenzi who engaged in death-feigning behavior. They noted, on April 10, 2011 at 11:30 A.M., a semi-adult Boiga [ Toxicodryas ] blandingii fell from the wooden roof of a golf club building from a height of 3 m onto a lawn. The snake was caught for the purpose of photography. It had apparently swallowed two smaller prey, and bats peeped excitedly from the hollow roof. The animal was carefully photographed for about ten minutes, remaining calm and not attempting to bite. Suddenly it opened its mouth and froze all movements. It couldn’t be made to move again. Even turning on its back did not lead to any reaction, and the snake remained motionless, as if dead, with its mouth wide open. Slight movements of the trachea could be registered.
Reproduction. Leston & Hughes (1968) documented a large female from Ghana that was found in early July with eggs. Groves (1973) noted that in 1969, a captive adult female from Liberia laid seven eggs on 5 June and another clutch of five eggs between 16–20 August; the following year she laid 10 eggs on 23 May and seven more from 10–13 August. In 1971, this female laid nine eggs ( 47.3–60.4 mm long, 23.2–28.7 mm wide, and 21–26 g in weight) on 1 May and six more on 30 July. Total length of hatchlings from 1971 ranged from 510–560 mm, which were described as “uniformly vicious.” Butler & Reid (1990) found wild hatchlings ( 590–630 mm; presumably total length) in Nigeria in January, March and December; one female contained three eggs in September. Luiselli et al. (1998a) found that in Nigeria, juvenile snakes were encountered in late April and late May; two adult females containing shelled follicles were found in March ( 1945 mm total length with 6 eggs) and April ( 1680 mm total length with 4 eggs). Akani et al. (2007) documented two Nigerian snakes— one female ( 188 cm total length) contained five eggs in June, and another individual ( 176 cm total length) contained seven eggs in March.
Habitat. Mocquard (1896:59) noted the species “habite les rochers” [inhabits rocks] in Guinea. Pasqual (1962) remarked that this species favors thatched roofs in Nigeria. Knoepffler (1966) described a specimen (MBG 0519) that was collected from a cave in Gabon where an enormous colony of bats and rockfowl ( Picathartes oreas ) were nesting, but the snake’s stomach was empty. Leston & Hughes (1968) found several snakes in trees of a cocoa farm in Ghana. Roux-Estève (1969) documented a subadult that was taken from a palm tree near a grove in Ivory Coast. Hughes (2000) explained how many authors restricted the species to forest (e.g., Perret 1961; Isemonger 1962; Roux- Estève 1969; Hughes 1983), but in fact, it occurs in several different habitats including savanna and urban areas, always seeming to seek out places with trees.
Mertens (1941) documented a snake that was found in a bunch of bananas on Bioko Island, Equatorial Guinea. Cansdale (1955) suggested that in Ghana, the black phase occurs in clearings, whereas the brown one inhabits farms and forests, but Hughes (2000) pointed out that this is likely an artifact of sampling or attributable to a higher rate of activity among males, which are usually black. Jones (1961:69) documented a snake that was living in an “asbestos roof” that was 30 feet [ 9.1 m] high in Sierra Leone. Cansdale (1965:43) later added that “both [color] forms are found equally everywhere.” Blackwell (1967) listed the species from forest, thickets, and gardens in Nigeria. Hughes & Barry (1969) listed the habitat as forest and wooded savanna in Ghana. Butler & Reid (1986) noted the species from forests, farms, and swamps in Nigeria. Hughes (1988:table1) listed the species from every major habitat in Ghana, including coastal thicket, rain forest, “moister ( Guinea) savanna, and drier ( Sudan) savanna.” Rasmussen (1997a) documented the species from gallery forests, collections of trees in grasslands, and hedges and solitary trees near human habitations. Luiselli et al. (1998a) encountered snakes in Nigeria from periodically flooded swamp forests, dryland forests, cultivated land near villages, suburbs of major urban areas, and mangroves, suggesting the species is a habitat generalist.
Rödel et al. (1999) recorded the species from gallery forest within savanna (including one individual who fell from a tree) in Ivory Coast. Greenbaum & Carr (2005) found an adult female in a tree in a village near dry forest in Guinea. Leaché (2005:table 1) listed the species from “semi-deciduous forest” in Ghana. Trape & Mané (2006) remarked that the species prefers very large trees. Akani et al. (2007) documented the species in oil palm plantations in Nigeria. Chirio & LeBreton (2007) noted the species from forests, savanna-forest mosaic, and high-elevation ( 1400 m maximum) savannas in Cameroon. Segniagbeto et al. (2011:331) recorded the species from all “ecological regions” in Togo, but it was most common in forest. Auliya et al. (2012:265) documented snakes from islands of Guinea-Bissau “on the roadside amid dense shrubs of secondary bush and palm vegetation” at night, and another individual from an oil palm approximately 8 m above the ground. Akaffou et al. (2017) listed the species from rubber tree ( Hevea brasiliensis ) plantations in Ivory Coast. Pauwels et al. (2017a, 2019 a, 2020) noted the species can be found in Gabon in several types of habitats, including savanna, forest, cities, caves, and even beaches. On the Guinean side of Mt. Nimba (ca. 1000 m elevation), snakes were frequently seen near a mining camp that is in humid savanna near rainforest (MOR, pers. obs.).
Geographic distribution. Based on molecular data from Allen et al. (in press) and patterns of our morphometric data ( Table 1), we hypothesize that this species occurs west of the confluence of the Congo and Ubangi Rivers from Guinea-Bissau to southwestern Central African Republic ( CAR) and eastern Republic of Congo.
Venom. Based on a specimen from Cameroon, Taub (1967) described the histological morphology of the Duvernoy’s gland. Groves (1973:108) described several “crudely performed” experiments to document the effects of captive snakebites ( one female originating from Liberia, one male of unknown origin, and their presumptive offspring) on mice, guinea pigs, and a capuchin monkey, all of which resulted in death after 16–110 minutes. Based on venom collected from one of the latter snakes, Levinson et al. (1976:311) documented a LD 50 value of 106 μg per mouse ( 3.23 mg /kg sensu Weinstein & Kardong 1994:table 3), and powerful neurotoxic components that cause “irreversible inhibition of the chemical excitatory mechanism in the postsynaptic membrane of the neuromuscular junction.” Based on venom collected from two snakes from Ghana, Weinstein & Smith (1993) estimated LD 50 values ranging from 2.85–3.55 mg /kg in mice. Immunological cross reactivity with elapid venom antisera was demonstrated, but Weinstein & Smith (1993) did not distinguish between samples originating from West Africa (i.e., T. blandingii ) or East Africa (i.e., T. vexator sp. nov.). Greene (1997) suggested the neurotoxic venom components of this species are as toxic as some elapids. This contention was supported by the study of Broaders & Ryan (1997) who studied venom from snakes originating from Togo and Benin, and demonstrated the presence of acetylcholinesterase, which is common in many elapid venoms. In venom from West African T. blandingii, Broaders et al. (1999) documented acetylcholine receptor binding activity. Amri & Chippaux (2012:142) described the mild bite of a captive adult male originating from Togo, including muscle pain, cramps, and “troubles de la sensibilité” [sensitivity trouble].
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Toxicodryas blandingii
| Greenbaum, Eli, Allen, Kaitlin E., Vaughan, Eugene R., Pauwels, Olivier S. G., Wallach, Van, Kusamba, Chifundera, Muninga, Wandege M., Aris- Tote, Mwenebatu M., Mali, Franck M. M., Badjedjea, Gabriel, Penner, Johannes, Rödel, Mark-Oliver, Rivera, Jacqueline, Sterkhova, Viktoria, Johnson, Grant, Tapondjou, Walter P. & Brown, Rafe M. 2021 |
Boiga blandingi occidentalis : Stucki-Stirn (1979:377)
| Stucki-Stirn, M. C. 1979: ) |
Boiga blandingi subfulva : Stucki-Stirn (1979:381)
| Stucki-Stirn, M. C. 1979: ) |
Dipsas globiceps var. tumboensis : Müller (1885:688)
| Muller, F. 1885: ) |
Dipsas
| Dumeril, A. H. A. 1859: 212 |
Toxicodryas
| Hallowell, E. 1857: 60 |
Dipsas fasciata : Fischer (1856:84)
| Fischer, J. G. 1856: ) |
Dipsas valida : Fischer (1856:87)
| Fischer, J. G. 1856: ) |
Dipsas globiceps : Fischer (1856:89)
| Fischer, J. G. 1856: ) |