Therophilus rugosus (Turner)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2887.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5292630 |
|
persistent identifier |
https://treatment.plazi.org/id/16742D5F-FFB6-6A73-FF34-F950FB0F6E78 |
|
treatment provided by |
Felipe |
|
scientific name |
Therophilus rugosus (Turner) |
| status |
|
( Figs 3D View FIGURE 3 , 5H View FIGURE 5 , 14A–D View FIGURE 14 , 21A View FIGURE 21 )
Agathiella rugosa Turner, 1918a: 112 [examined]. Holotype BMNH ♀; Type locality: Eaglehawk Neck , Tasmania.
Agathiella rugosa (Turner) ; Parrot, 1953: 198 [catalogue].
Agathis rugosa (Turner) ; Shenefelt, 1970b: 353 [catalogue, generic transfer].
Bassus rugosus (Turner) View in CoL ; Yu et al., 2005 [catalogue, generic transfer].
Therophilus rugosus (Turner) ; Stevens et al., 2010: 21 View Cited Treatment [catalogue, generic transfer].
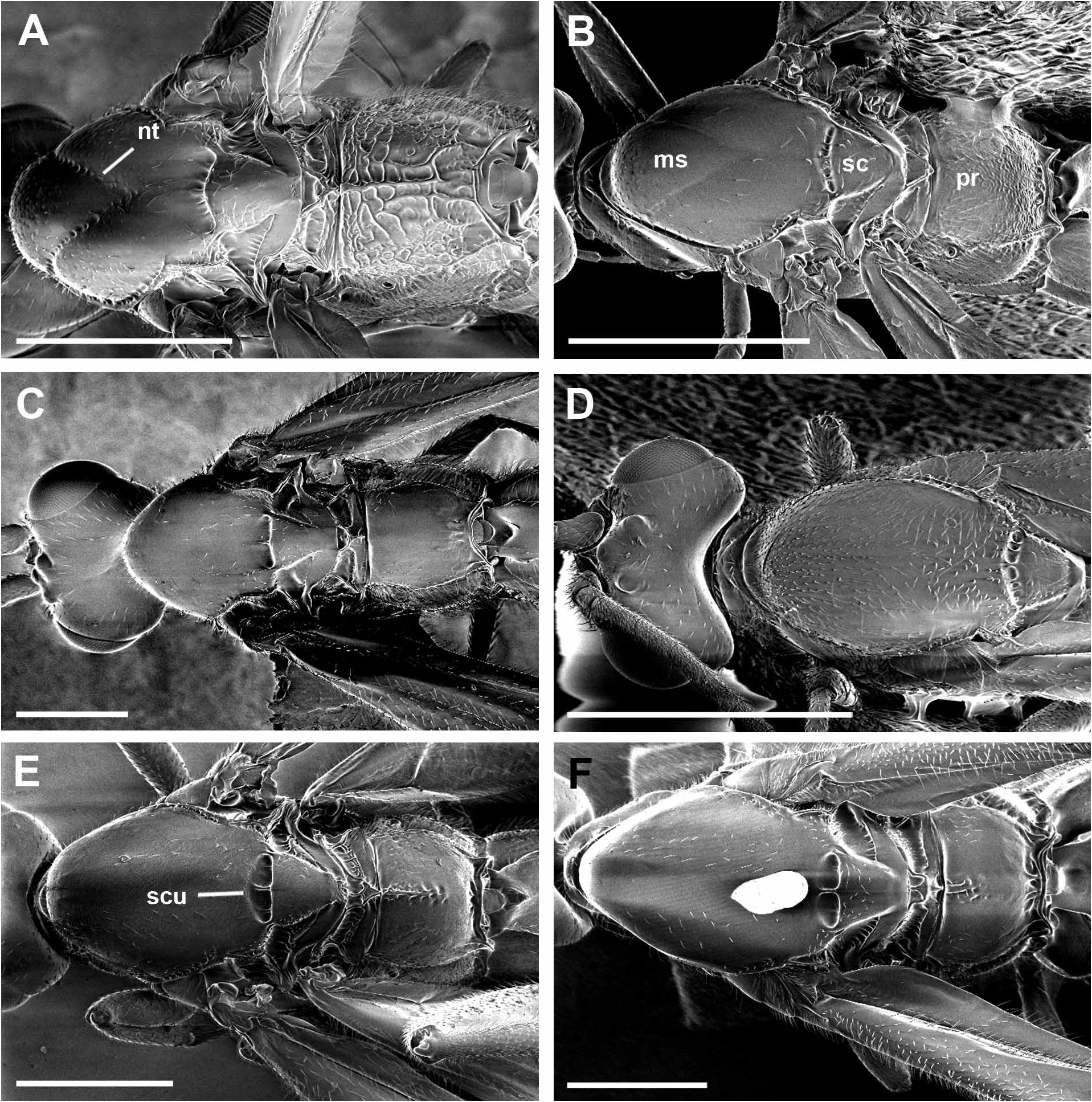
Diagnosis. Broad carina between antennae; notauli absent anteriorly; sternalus distinctly scrobiculate; hind tibia with distinct black and white bands; propodeum extensively rugose; median T1 and T2 with faint granulate sculpturing; ante-ocular pit small and indistinct; distance of lateral ocelli from median ocellus marginally greater than median ocellus diameter; posterior margin of head, when viewed dorsally, broadly excavated, depth of excavation greater than diameter of median ocellus; with BROW colour pattern.
Description (female). Body length 5.4 (3.6–5.4) mm; ovipositor 5.4 (2.3–5.4) mm; head mostly orange, except black dorsally; antenna black; mesosoma mostly orange except posterior sclerites, propodeum, metapleuron, mesepimeron and ventral mesopleuron black (metanotum and most of mesopleuron sometimes black); fore leg coxa orange, fore trochanters and tarsomeres darker, fore femur and tibia pale yellow (entire fore leg sometimes uniform yellow-orange with dark claws); mid leg coxa to basal femur black to dark brown, distal half of femur yellow, tibia with narrow pale band basally, then narrow dark band, followed by broad pale band medially, then darker distally, tibia spurs pale; hind leg femur black, tibia with pronounced pale and dark banding, starting basally with narrow pale band, then narrow dark band, followed by a broad pale band medially then darker distally (basal narrow dark band may not fully encircle tibia); median T1 mostly black except for thin white bands on all margins (T1 sometimes entirely or mostly white); median T2 mostly black except white anterior and antero-lateral margins (T2 sometimes entirely or mostly white); T3 mostly black but with white incursions antero-laterally (T3 sometimes black posteriorly only); lateral tergites of T1 and T2 white; S1 and S2 mostly white except small dark patch medial S1 (S1, S2, and S3 sometimes entirely white); remainder of metasoma black to dark brown.
Head width 1.1 (0.8–1.1) mm, length 0.6 mm, height 0.9 mm; eye width 0.3 mm, length 0.4 mm, height 0.5 mm; head triangular in anterior view but not extremely so; inter-orbital distance 0.7 mm; median ocellus diameter 0.08 mm; distance between lateral ocelli and median ocellus 0.1 mm; distance between lateral ocelli 0.17 mm; single, broad carina between antennae; ante-ocular pit small and indistinct; antenna with 30–32 flagellomeres; clypeus width 0.4 mm, height 0.2 mm; malar space height 0.2 mm; distance from ventral eye margin to latero-ventral mouth margin 0.3 mm; labial palpomere 3 length 0.2x labial palpomere 4 length (lengths 0.02 and 0.12 mm, respectively); labial palpomere 2 length 0.1 mm; posterior margin of head, when viewed dorsally, broadly excavated (incursion 0.1 mm); posterior genal margin distinctly carinate with no expansion ventro-posteriorly.
Mesosomal width 0.9 (0.8–1.0) mm, length 2.2 (1.4–2.2) mm, height 1.5 (0.9–1.6) mm; pilosity mostly 0.05– 0.08 mm in length and sparse, particularly dorsally, except for distinct dense setal field on metapleuron and mesepimeron, extending marginally onto mesopleuron and propodeum, where setae long (0.1–0.15 mm) and thick; antescutal depression distinct; subpronope distinct, bordered posteriorly by pair faint, short carinae that do not extend to anterior margin of pronotum; notauli absent anteriorly; scutellar sulcus divided into 4 main pits by 3 distinct longitudinal carina, medial carina largest, smaller pits on lateral margins, anterior wall sloped, particularly medially, posterior wall steeper, nearly vertical; propodeal surface extensively rugose; propodeal spiracle ovoid (maximum width 0.06 mm); suture line between metapleuron and propodeum delineated by scrobiculate groove; hind coxal cavities closed to metasomal foramen by hind coxal bridge (minimum width 0.08 mm) with carinate margins; sternalus distinctly scrobiculate and relatively straight and horizontal posteriorly, slight upward curvature anteriorly; metapleural with white reflective setal field; surface smooth except for punctation associated with setae.
Legs with basal lobe of all claws small, rounded triangular protrusions; mid tibia with 3 apical and 3 preapical spines; preapical spines on anterior surface in distal half of tibia; hind tibia with 4 apical and 6 preapical spines; preapical spines clumped just basal to apical spines.
Both fore and hind wings lightly infuscate (sometimes clear); fore wing maximum width 1.5 (1.1–1.6) mm, length 4.6 (3.2–4.6) mm; cell 1–Rs petiolate and triangular, reduced in size, maximum distance across cell marginally greater than width of cell petiole vein (maximum distance across cell 1-Rs equal to or less than width of cell petiole vein); petiole of cell 0.1 mm long; basal one-third of M+Cu lightly pigmented (sometimes most of M+Cu unpigmented); hind wing maximum width 1.0 (0.6–1.1) mm, length 3.8 (2.6–3.8) mm.
Metasomal length 2.0 (1.5–2.2) mm, maximum width 0.8 mm; most dorsal surfaces smooth except median T1 and T2 with faint granulate sculpturing; T1 with paired, sinuate carinae latero-medially in anterior half; T1 median area triangular in dorsal view, T1 length 0.7 (0.5–0.8) mm; anterior width 0.3 (0.2–0.4) mm, posterior width (maximum) 0.7 (0.4–0.8) mm; T2 with medial transverse groove; T2–T3 boundary marked by shallow groove.
Male. As for female except for genitalia.
Holotype: ♀, ‘ Eaglehawk Neck, S.E. Tasmania. Feb. 12–Mch 3, 1913. R.E. Turner 1913–212’ ( BMNH).
Other material examined. Australian Capital Territory: 5 ♀, 4 ♂, Canberra , 16.I–16.III.1979, Malaise trap, C.R. Tidemann ( ANIC) ; Lord Howe Island : 1 ♀, 20.II–6.II.1957, Z.R. Liepa ( BPBM) ; New South Wales: 2 ♀, Braidwood, Glenmore Rd , 17–29.XII.2005, C.J. Stephens, malaise trap, exotic/native garden blend in pasture setting ( ANIC, WINC) ; 2 ♀, Eden, Bungo St. , 21–27.XII.2005, C.J. Stephens, malaise trap, exotic/native garden blend nr eucalypt forest ( ANIC, WINC) ; Northern Territory: 1 ♀, 30 km S of Alice Springs , 3.XI.1974, on Cassia pleurocarpa, E.M. Exley & R.I. Storey ( UQIC) ; Queensland: 1 ♂, D.P.I. Research Station, Gatton , 14– 21.IX.1981, Malaise trap ( QDPI) ; 1 ♀, Lower Beechmont , 1–4.I.1982, G.J. & A. Holloway ( AMSA) ; 1 ♂, Kuranda, Russet Park , 460 m, 8.XI.1987, T.W. Davies ( CASC) ; South Australia: 1 ♀, Gladstone , reared ex. larva of Etiella behrii collected 11.II.1959, emerged 2.III.1959, D.J. Taylor ( WINC) ; 1 ♀, Tintinara , ex. larva of Etiella behrii collected 9.I.1962, D.J. Taylor ( WINC) ; 2 ♀, Kangaroo Island, Flinders Chase National Park , 25.XII.1989 – 6.I.1990, R. Wharton ( TAMU) ; 1 ♂, Glenroy National Park , 14 km NE Coonawarra, ex. light brown apple moth, Epiphyas postvittana , neonates put out on sentinel plants 28.XII.2004, emerged 17.I.2005, C. Paull ( WINC) ; Tasmania: 1 ♂, Hollow Tree , 42°32’S 146°56’E, 12.II.1992, sweeping roadside vegetation, mostly grasses, D.S. Horning, Jr ( ANIC) GoogleMaps ; Victoria; 1 ♀, 1 ♂, La Trobe River Survey, 10–31.X.1973, U/stream A.P.M. Gen. Terrestrial ( MVMA) ; Western Australia: 1 ♀, Perth , 8.IV.1936, A.L. Raymond, B.M. 1936–1+29 ( BMNH) ; 1 ♀, Darlington, Perth , II.1977, G.H. Lowe ( WAMP) ; 1 ♀, Eneabba (29°49’S 115°16’E, 4.IX.1981, R.P. McMillan ( WAMP) GoogleMaps ; 1 ♀, Kelmscott, Perth , 32°07’S 116°01’E, 5.IV.1984, P. Hutchinson ( UQIC) GoogleMaps ; 1 ♀, Mount Lawley, Perth , 13X.1986, J.M. Waldock ( WAMP) ; 1 ♀, 11 km SE Eurardy Homestead , 27°39’17’’S 144°43’25’’ E, 25.X.2000, T.F. Houston & O. Muellar ( WAMP) GoogleMaps .
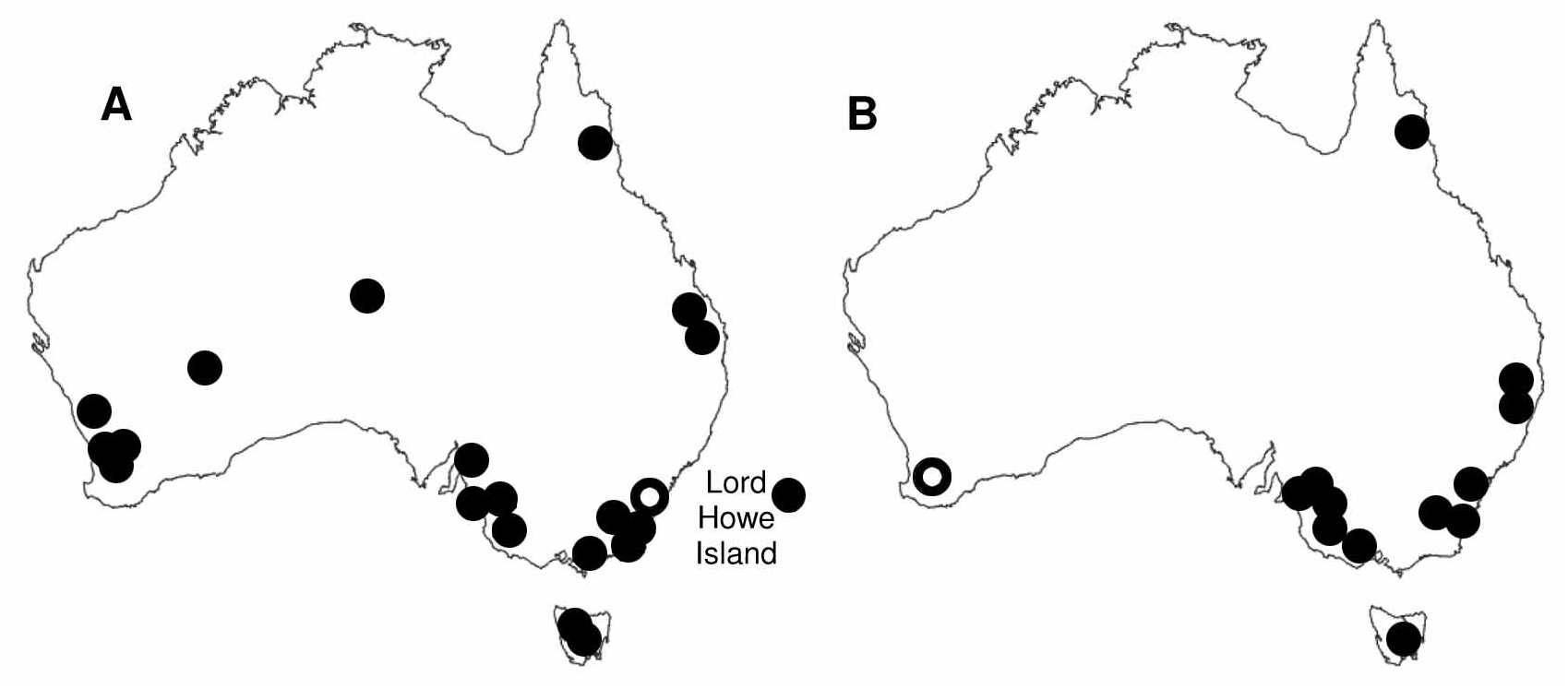
Comments. Therophilus rugosus exhibits a similar BROW colour pattern to T. tricolor , including the black and white banding pattern on the hind leg. However, T. rugosus can be readily distinguished by the extensive rugose sculpturing of the propodeum compared with only faint sculpturing on T. tricolor . Therophilus rugosus is one of the most abundant and widespread species that is recorded throughout Australia, including Lord Howe Island, from the arid Eyrean to the temperate south-western, Kosciuskan, Tasmanian, tropical/sub-tropical Timorian and Torresian biogeographic regions ( Fig. 21A View FIGURE 21 ). Known host records ( Table 2) include two native pest lepidopteran species, Etiella behrii and Epiphyas postvittana .
The holotype is in relatively good condition except both antennae missing all flagellomeres.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Therophilus rugosus (Turner)
| Stevens, Nicholas B., Austin, Andrew D. & Jennings, John T. 2011 |
Agathis rugosa (Turner)
| Shenefelt, R. D. 1970: 353 |
Agathiella rugosa
| Turner, R. E. 1918: 112 |