Polydiscia deuterosminthurus, Baquero, Enrique, Moraza, Maria Lourdes & Jordana, Rafael, 2003
|
publication ID |
https://doi.org/ 10.5281/zenodo.156331 |
|
DOI |
https://doi.org/10.5281/zenodo.6275640 |
|
persistent identifier |
https://treatment.plazi.org/id/1D54879F-FFE6-FFAE-FEC3-81CEFA94C766 |
|
treatment provided by |
Plazi |
|
scientific name |
Polydiscia deuterosminthurus |
| status |
sp. nov. |
Polydiscia deuterosminthurus sp. nov. ( Figs 113 View FIGURES 1 2. 1 View FIGURES 3 6 View FIGURES 7 13 )
Typehost: Deuterosminthurus bisetosus sp. nov.
Typelocality: SPAIN, Navarra, Otazu (UTM coordinates 30TWN993375). Typespecimens: Holotype (larva) collected ex D. bisetosus sp. nov. from Genista hispanica . MZNA0031001 (mounted, permanent slide), 28 May 1999, E. Baquero and R. Jordana leg. Paratypes: two specimens mounted in permanent slide (MZNA0031002 and MZNA0031003), three specimens in ethyl alcohol.
Additional material: Same locality, MZNA 00309, 26 May 1999, E. Baquero & L. Hernández leg. (nine larvae on SEM stub). MZNA 00353, 9 May 2000 (three larvae in ethyl alcohol), MZNA 00362, 26 May 2000 (eight larvae in ethyl alcohol), MZNA 00389, 30 May 2001 (five larvae in ethyl alcohol), E. Baquero and R. Jordana leg.
Zoolog. Inst. Innsbruck ( Austria): one slide labelled as ‘ Polydiscia squamata Trombidiiden Larve. Leg. et det. Dr. Methlagl. Gaaden b. Wien TYPE’. Designated 'lectotype' by VercammenGrandjean (1972: 237).
Material deposited: MZUN (Museum of Zoology, University of Navarra).
Diagnosis. Larva with the following features: fD = 4 – 4 – 4 – 4 2 = 18; fV = 2 – 2 2u 2 = 8; fnCx = 2 – 1 – 2; fnTr = 1 – 1 – 1; fnFe = 6 – 7 – 6; fnGe = 4 – 4 4; fnTi = 9 – 9 9; fnTa = 25 – 21 20; fSol = I(0 – 3 – 4 –1), II(0 – 4 – 2 – 1), III (0 – 1 – 1 0); f = I(1 1), II(1 0), III(0 0); f = 2 – 2 – 0; f = 1 – 1 – 0; fPp = 0 – B – B – BNNN – 6B2; Ip = 697; Tarsus III with developed pretarsus; fSc = PL> S> AL> AM, AP subigual to AM, AA <SB.
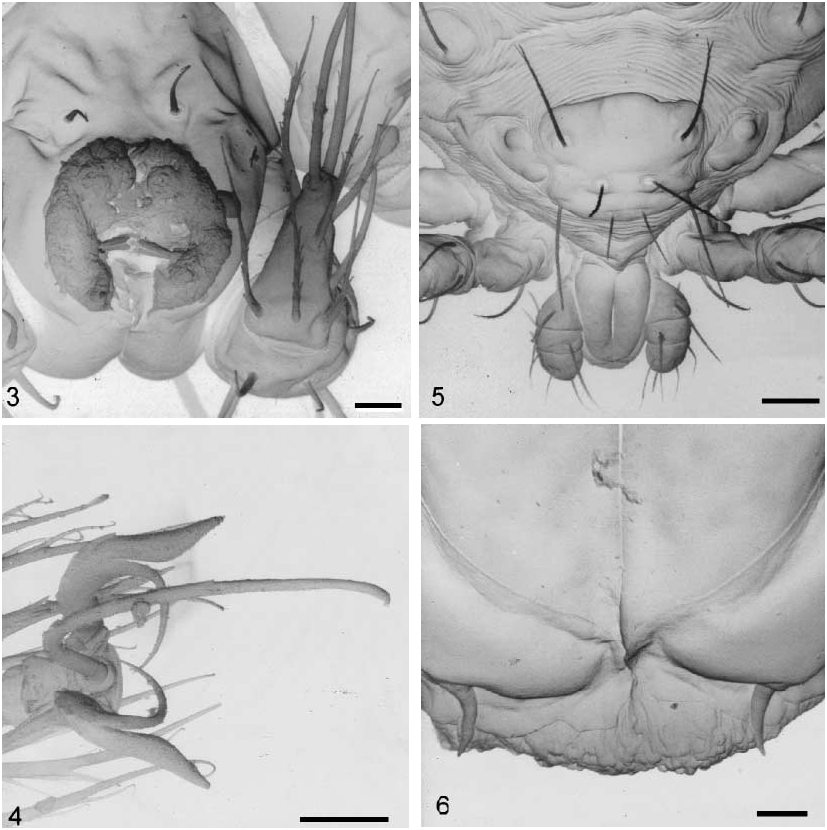
Description ( Figures 113 View FIGURES 1 2. 1 View FIGURES 3 6 View FIGURES 7 13 ). Larva (holotype). Measurements of holotype, the mean, range, and number of other specimens measured in parentheses; when the number is three, the measurements belongs to the paratypes. Colour in life red; white in ethyl alcohol. Idiosoma ( Figures 2 View FIGURES 1 2. 1 , 5 View FIGURES 3 6 , 8 View FIGURES 7 13 ). Holotype 267 m long (240, 140290, 25), 205 m wide (160, 120 205, 25).
Dorsum: Two eyes similar in size, diameter 9 m (11, 911, 3) on each side of prodorsal sclerite set in an oval sclerite, 29 m long (29, 2830, 3), 15 m wide (1115, 3), 3 m larger than each eye (3, 3, 3). Dorsum with nine pairs of weakly barbed setae ( Figure 7 View FIGURES 7 13 ) ranging in length from 40 m to 47 m, all arising from large platelets ranging in size from 20 x 31 m to 20 x 28 m; cupules ia, im, ip and ih present.
Venter: Two pairs of branched sternal setae, 19 m and 15 m respectively, located on their respective coxal field. Three pairs of pseudanal setae: ps1 37 m long, ps2 36 m long and ps3 30 m long ( Figure 8 View FIGURES 7 13 ). Anal sclerite 30 m long (30, 3032, 3) by 14 m wide (13, 1415, 3) ( Figure 8 View FIGURES 7 13 ).
Prodorsal sclerite. Shape roughly trapezoidal with anterior and posterior margins convex in the middle region, lateral margins deeply concave, with punctated surface; setae PL>S>AL>AM (AM and SS trichobothridial setae weakly barbed; Figures 5 View FIGURES 3 6 , 9 View FIGURES 7 13 ). Measurements of prodorsal sclerite and its setae in Table I.
Gnathosoma ( Figures 3, 58 View FIGURES 3 6 View FIGURES 7 13 ). Pedipalps: trochanter 18 m; femur 10 m; genu 13 m; palpal tibiatarsus 16 m. Palpal tibia and tarsus fused. Palpal setal formula: 0 B B BNNN 6B2; tibial claw (following to VercammenGrandjean, 1972) 24 m long (21, 1824, 3) in apical position, resembling the dorsoapical slightly barbed tarsal eupathidia 22 m long (22, 2022, 3). Adoral setae ( Figure 6 View FIGURES 3 6 ) shorter and wider than pair of subcapitular setae sc1. Chelicera with an oblong shaft, 52 m long (51, 5152, 2) by 19 m wide (18, 1819, 2); chelostyle 10 m long (10, 911, 2) with a deep longitudinal groove and without teeth ( Figure 3 View FIGURES 3 6 ).
Legs ( Figures 4 View FIGURES 3 6 and 1113 View FIGURES 7 13 ). Femora of all legs divided; pretarsi IIII with a thin and curved sickleshape empodium; paraxial and antiaxial smooth claws with similar shape, divided in two branches: paraxial branch longer, thinner and sharply curved; antiaxial branch foliate and sinuous. Legs I length 245 m (229, 207245, 3); coxal field with 2 branched setae (2B); trochanter 1B; basifemur 1B; telofemur 5B; genu 4B, 3 22 m, 1 microseta 4 m; tibia 9B, 4 22 m, 1 k 4 m; tarsus 1 27 m, 1famulus 4 m, 2 eupathidia with setules (h (= ST1 33 m, pST1 13 m) and p 22 m) and 25 B. Legs II length 219 m (218, 215219, 3): coxal field with 1 branched seta (1B); trochanter 1B; basifemur 2B; telofemur 5B; genu 4B, 1 25 m, 1 microseta 5 m; tibia 9B, 2 19
m; tarsus 1, 1famulus, 2 eupathidia with setules (h 29 m, 7 m and p 22 m) and 21 B. Legs III length 245 m (251, 245259, 3); coxal field with 2 branched setae (2B); trochanter1B; basifemur 2B; telofemur 4B; genu 4B, 1; tibia 9B, 1; tarsus 20 B.
TABLE I. Polydiscia deuterosminthurus sp. nov. Measurements of prodorsal sclerite and its setae.
PT= Paratype; LT= Lectotype; AM=anteromedial setae (trichobothridial); SS= posteromedial setae (trichobothridial); AL= anterolateral setae; PL= posterolateral setae; AA= distance between the insertion of setae AM; SB= distance between the insertion of setae S; AW= distance between the insertion of setae AL; PW= distance between the insertion of setae PL; ASB= distance between the anterior margin of the prodorsal sclerite and the base of botrichial setae S; PDB= distance between the posterior margin of the prodorsal sclerite and the base of botrichial setae S; SD=length of the prodorsal sclerite; SD= ASB+PSB; AP= distance between the insertion of setae AL and PL; pa=legI lengh; pm=leg II lengh; pp= leg III lengh; Ip=pa+pm+pp; T13= lenght of tarsus I, II and III.
Discussion. We had the opportunity to study a lectotype of the type species (specimen also studied by VercammenGrandjean in his paper of 1972). However, it shows evidence of having been manipulated and actually lacks both pairs of trichobothria. Our observations expose errors of observation or interpretation of several characteristics given by VercammenGrandjean for the species. The missing setae enable us to confirm an important characteristic of this species and we were forced to follow VercammenGrandjean´s description and measurements of these setae.
Although VercammenGrandjean (1972) indicate a palpalsetal formula for P. squamata of (B)(B)(N).N.N, the type studied shows a formula similar to that of P. deuterosminthurus sp. nov. and on the coxae, all the setae were described as nude (they are clearly branched setae). In leg I, P. squamata has two genualae and although Vercammen Grandjean (1972) indicate two tibialae and 21 branched setae in the tarsus, the type specimen has four on tibia and 25B and p with setules in the tarsus.
P. deuterosminthurus sp. nov. can be separated from P. s q u a m a t a Methlagl, 1928 by the following characteristics: (1) besides length difference of the prodorsal sclerite (AD), AA, PW, AP (see table I), in P. deuterosminthurus sp. nov. setae PL>S>AL>AM versus PL<S>AL<AM. ( VercammenGrandjean, 1972) in P. s q u a m a t a; (2) shorter length of legs IIII in P. deuterosminthurus sp. nov., Ip = 710 (697, 682710, 3) versus Ip = 771 (742800, 2) in P. squamata ; (3) tarsus II shorter in P. deuterosminthurus sp. nov., T2 = 57 (56, 56 57, 3) versus T2 = 64 (6364, 2) in P. squamata ; (4) leg I with 3 in P. deuterosminthurus sp. nov. versus 2 in P. squamata .
The specimens obtained by Methlagl of P. squamata were collected freeliving in vacant lot and this species´s host is yet unknown.
Biology. With the capture of the larvae of P. deuterosminthurus sp. nov. parasitizing D. bisetosus sp. nov., the first host association of this genus is established.
Concerning the location of the mite on the host, the majority were found on the dorsal body surface, with the gnathosoma embedded in the posterior area of the articulation between head and prothorax ( Figure 1 View FIGURES 1 2. 1 ). This location agrees with that seen by Greenslade & Southcott (1980) for the erythraeid parasites from Corynephoria observed in Australia.
Although Greenslade & Southcott (1980) wrote that ‘parasitism on the Collembola is unknown although animals seemed moved normally and on microscopic examination appear unaffected’, we observe that the physiological effect on the host may be lethal since the infested hosts show a high degree of dehydration, with a lack of antennal turgescence in such a way that these appendices rest folded back. The mites are easily separated from the host and although light scars were observed, the degree of injury to the host cuticle was not great.
When parasitic mite densities are high, the effect on collembolan populations could be significant ( Greenslade & Southcott, 1980). In our population the mean intensity of infestation is 1, although several Collembola bear two mites. The prevalence, that is, number of individuals of a host species infested divided by the number of hosts examined ( Margolis et al, 1982), is around 10%. The relationship between parasite and host size is approximately 3:1.
P. deuterosminthurus sp. nov. seems to have an annual cycle, with a larval parasitic instar during the spring. It shows a high specificity for the Collembola species later described, since the mite has been not found on other Collembola and arthropods living together with the parasite species.
The sampling was extended over a period of several weeks after the first capture (May 1999). Adult specimens of the mites were never found. D. bisetosus sp. nov. and larvae of P. deuterosminthurus sp. nov. were not found on plants after the first fortnight of June, the moment of the year when temperatures increase and the environmental humidity does not allow the presence of Symphypleona on the vegetation. Further specimens of D. bisetosus sp. nov. and P. deuterosminthurus sp. nov. were not captured until May of 2000 and 2001. Attempts to rear the mite in the laboratory in order to follow the postlarval instars failed.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |