Magnolia salicifolia, (Siebold & Zucc.) Maxim. (Siebold & Zucc.) Maxim.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2015.02.025 |
|
DOI |
https://doi.org/10.5281/zenodo.10524079 |
|
persistent identifier |
https://treatment.plazi.org/id/2028C32F-B93E-9A0C-3B1F-A41B150AF859 |
|
treatment provided by |
Felipe |
|
scientific name |
Magnolia salicifolia |
| status |
|
2.1. Phenolic glycosides detected in tepals of M. salicifolia View in CoL
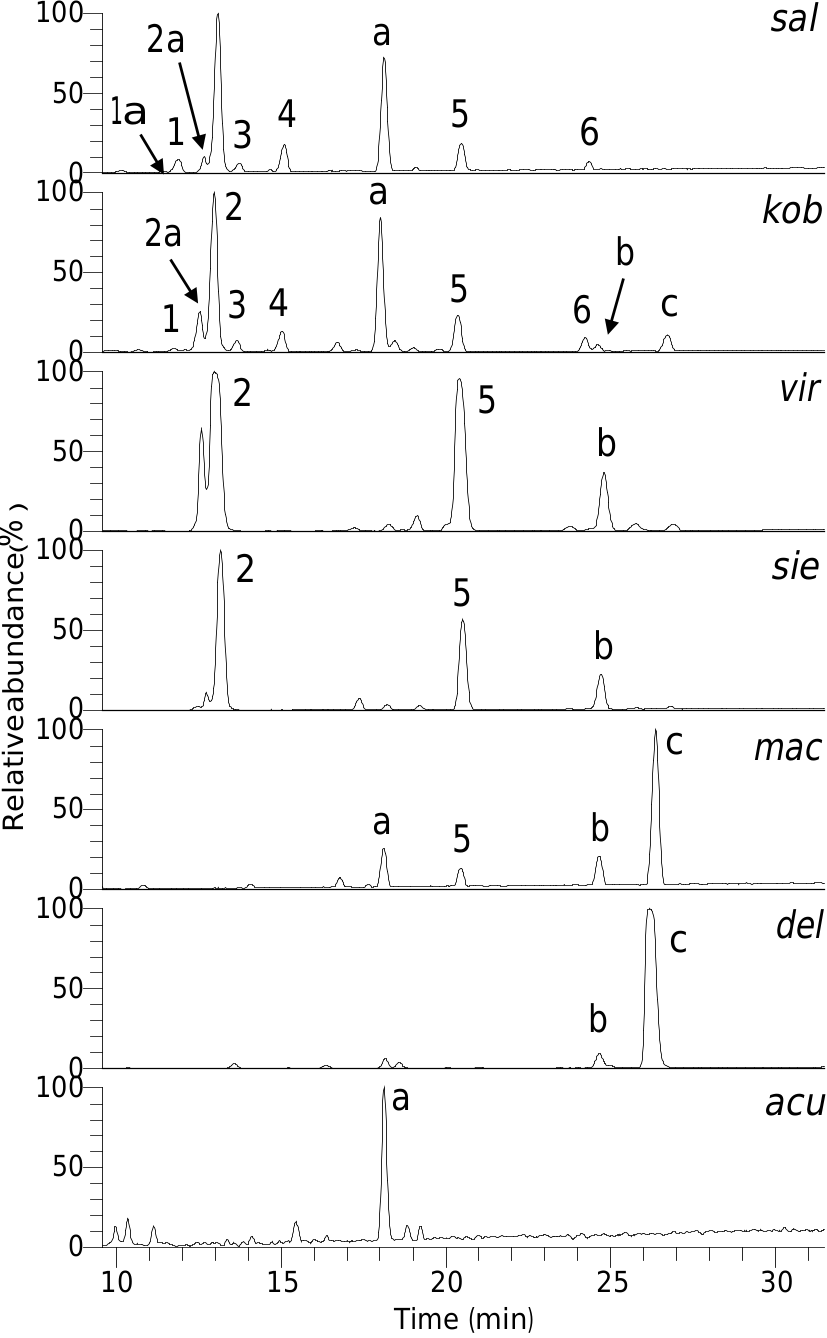
LC–UV–MS analysis of an 80% CH 3 OH extract of the tepals of M. salicifolia revealed several abundant UV absorbing components ( Fig. 2 View Fig ). The UV and serial mass spectra of these suggested they were phenylethanoid glycosides ( 1–6) and the flavonol glycoside quercetin 3- O -rutinoside. The latter, together with the 3- O -rutinosides of kaempferol and isorhamnetin, present as minor components of the extract, were identified by the mass spectrometry methods described by Kite and Veitch (2011).
The phenylethanoid glycoside 5 was identified as verbascoside by chromatographic and mass spectrometric comparison with a standard. Verbascoside is an acylated glycoside of 3,4-dihydroxyphenylethanol (= hydroxytyrosol; M = 154), the acylating acid being caffeic acid ( M = 180). The presence of these two phenolic moieties in 1–4 was suggested from ion trap MS/MS of their doubly deprotonated molecules [M–2H] 2 —, which gave singly charged fragments at m / z 135.0456 (C 8 H 7 O 2), 161.0248 (C 9 H 5 O 3) and 179.0354 (C 9 H 7 O 4), expected for [(hydroxytyrosol–H 2 O)–H] —, [(caffeic acid–H 2 O)–H] — and [caffeic acid–H] —, respectively. Neither 5 nor 6 generated doubly deprotonated molecules but, following ion trap MS/MS, the singly deprotonated molecule of all of 1–6 showed a neutral loss of C9H5O3 (caffeic acid–H 2 O). Collision cell MS2 spectra of deprotonated 1–6 also gave [(caffeic acid–H 2 O)–H] — as the base ion. The sodiated molecules of 1–6 predominately showed a neutral loss of 146 (Rha) following ion trap MS/MS. Compounds 3, 4 and 6 had molecular masses that were each 146 Da greater than 1, 2 and 5, respectively, and the [(M–Rha) + Na] + ion of 3, 4 and 6 generated by MS/MS fragmented by further rounds of serial mass spectrometry in a similar manner to the sodiated molecules of 1, 2 and 5 (see Sections 3.6–3.13). This suggested that compounds 3, 4 and 6 were rhamnosyl derivatives of 1, 2 and 5, respectively.
During the isolation of 1 and 2, two other phenylethanoid glycosides, 1a and 2a respectively, became apparent. Compounds 1a and 2a were present in the initial extract, eluting just before 1 and 2, respectively ( Fig. 2 View Fig ), and their molecular masses were 14 Da less than 1 and 2, respectively. The sodiated molecules of 1a and 2a both lost 132 (pentose residue) to give an ion that fragmented in a similar manner to the [(M–Rha) + Na] + ion of 1 and 2, respectively. This suggested 1a and 2a were analogues of 1 and 2 in which the rhamnose at C-3 0 of the core glucose was replaced by a pentose sugar.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |