Palaeostigus Newton
|
publication ID |
https://doi.org/10.11646/zootaxa.4453.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:866690A9-0462-4892-AE29-9AAC623F87B3 |
|
DOI |
https://doi.org/10.5281/zenodo.5976958 |
|
persistent identifier |
https://treatment.plazi.org/id/2161879C-FF93-8A4D-FF7A-35C46552DC3E |
|
treatment provided by |
Plazi |
|
scientific name |
Palaeostigus Newton |
| status |
|
Palaeostigus Newton View in CoL
Palaeostigus Newton, 1998 (in Newton & Franz (1998)): 155. Type species: Mastigus palpalis Latreille, 1804 (des. Newton in Newton & Franz, 1998: 155). Note: the type species designation by Latreille (1802) for Mastigus makes that name a senior objective synonym of Australostigus , leaving Mastigus of Leleup (1968) without an available name (Newton & Franz 1998).
Mastigus of Leleup, 1968: 10 (misidentified, not Latreille, 1802). Type species: Mastigus palpalis Latreille, 1804 (des. Leleup, 1968: 7; invalid designation, species not originally included).
Diagnosis. Adults of Palaeostigus differ from remaining Mastigini in subtrapezoidal mesoventral intercoxal process with broad and nearly straight posterior tip ( Figs 191–192 View FIGURES 189–193 ); and distinct setose impression of mesoventrite ( Fig. 191 View FIGURES 189–193 ; si). Mature larva: antennae about twice as long as head capsule ( Figs 200–201 View FIGURES 200–201 ).
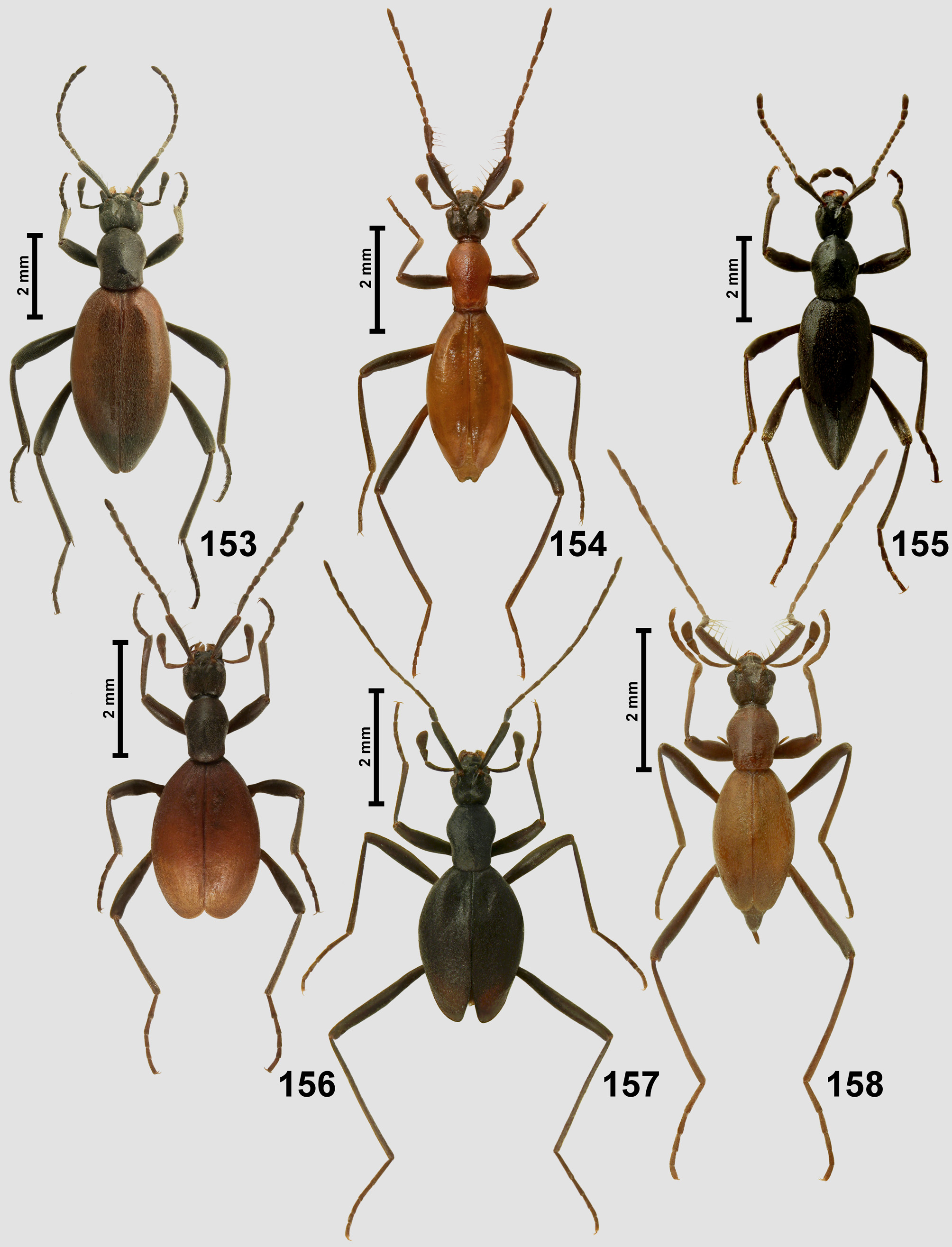
Characteristics. Adults. Body ( Figs 155–156 View FIGURES 153–158 , 159–160 View FIGURES 159–162 ) large, 3.40̄ 7.50 mm in length, uniformly black or nearly black or with dark brown or nearly black head and pronotum and reddish-brown or testaceous elytra; strongly convex, dorsally densely but finely setose, setae unmodified except for long and thick bristles on scape and pedicel.
Head capsule ( Figs 176–177 View FIGURES 176–179 ) divided into large and exposed anterior part and much smaller, subcylindrical 'neck' region retracted into prothorax and demarcated by distinct occipital constriction; 'neck' region much broader than half width of head. Anterior part of head flattened, subequal in width with prothorax, distinctly elongate, broadest near middle. Composite eyes dorsolateral, moderately large, composed of numerous small ommatidia, not projecting or weakly projecting from the silhouette of the head, broadly separated from mandibular bases. Vertex and frons divided by a distinct median longitudinal groove ( Fig. 176 View FIGURES 176–179 ; mg); vertex strongly transverse, convex at sides, with posterior margin nearly straight or slightly concave. Tempora much longer than eyes, weakly rounded. Frons between antennal insertions not forming a demarcated 'platform', weakly convex or flattened, anteriorly demarcated by a deep and very short frontoclypeal groove ( Fig. 176 View FIGURES 176–179 ; fcg) largely obliterated at sides. Clypeus very short and broad, broadly subtrapezoidal, with nearly straight sides, which are slightly convergent anterad or parallel. Antennal insertions ( Fig. 176 View FIGURES 176–179 ; ia) located anterodorsally, relatively narrowly separated. Gular plate ( Fig. 177 View FIGURES 176–179 ; gp) lacking sutures, indistinctly transversely reticulate; posterior tentorial pits ( Fig. 177 View FIGURES 176–179 ; ptp) oval, in front of broad and diffuse transverse impression demarcating 'neck' region ventrally; hypostomal ridges ( Fig. 177 View FIGURES 176–179 ; hr) arcuate, posteriorly reaching middle between anterior submental margin and posterior tentorial pits. Head finely to strongly punctate, densely setose ( Fig. 176 View FIGURES 176–179 ).
Antennae ( Figs 155–156 View FIGURES 153–158 , 159–160 View FIGURES 159–162 , 178 View FIGURES 176–179 ) long and slender, shorter than body; scape ( Fig. 178 View FIGURES 176–179 ; sc) 5–7 times as long as broad, thickened, much longer than head, with lateroventral (more ventral than lateral) emargination; pedicel ( Fig. 178 View FIGURES 176–179 ; pd) also conspicuously enlarged, much shorter and narrower than scape, typically 4–6 times as long as broad, broadening from narrow base to subapical region. Both scape and pedicel with two ventral longitudinal rows of several long and thick bristles with papillate insertions, area between bristles with variously densely distributed porous fields ( Fig. 179 View FIGURES 176–179 ; pf); antennomeres III–XI distinctly narrower than pedicel, elongate, each slightly thickened distad, basal stalks not exposed in intact beetles, basal rings absent or indistinct; antennomere XI elongate and indistinctly asymmetrical. Antennomeres covered with variously dense, long setae; surface of antennomeres smooth.
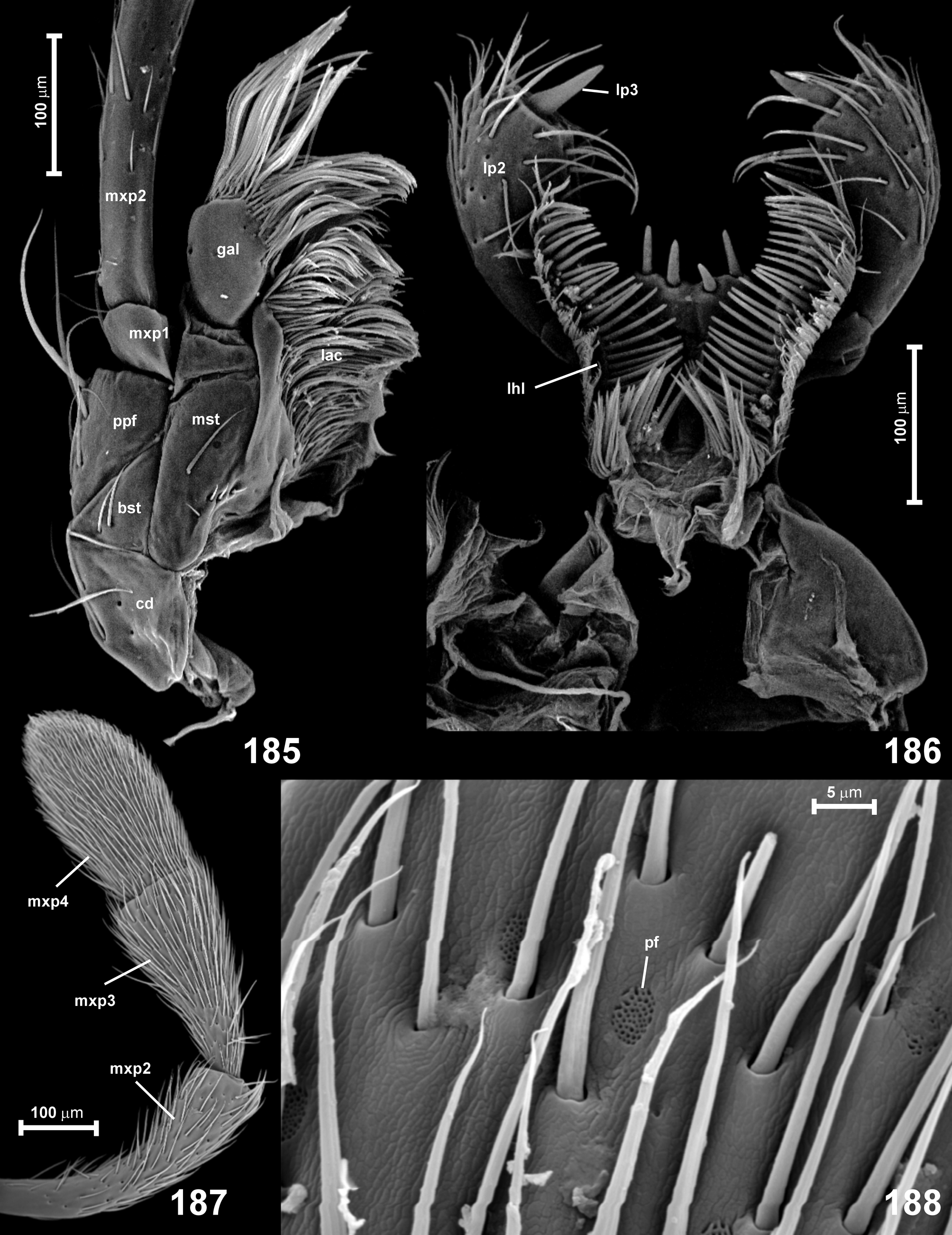
Mouthparts. Labrum ( Fig. 180 View FIGURES 180–183 ; lbr) strongly transverse, with lateral margins slightly convergent anterad and weakly rounded, and with anterior margin weakly concave, with a pair of broad and short sublateral teeth broadly separated at middle by a very shallow emargination, and with two partly irregular transverse rows of long and short setae; epipharynx ( Fig. 181 View FIGURES 180–183 ; eph) smooth, with dense lateral trichia. Mandibles ( Figs 182–183 View FIGURES 180–183 ) symmetrical, subtriangular and robust, each with one dorsal mesal tooth and a group of 2–3 ventral mesal teeth, setose prostheca ( Figs 182–18 View FIGURES 180–183 ; pst 3) present and long, its setae extending to dorsal and ventral surface of basal half of mandible. Maxilla (Figs 184–185) with large, long cardo ( Fig. 185 View FIGURES 185–188 ; cd); basistipes ( Fig. 185 View FIGURES 185–188 ; bst) subtriangular and elongate; mediostipes ( Fig. 185 View FIGURES 185–188 ; mst) large and sharply demarcated from lacinia ( Fig. 185 View FIGURES 185–188 ; lac) and galea ( Fig. 185 View FIGURES 185–188 ; gal), which are both elongate and each with conspicuously dense group of thin distal setae; palpifer ( Fig. 185 View FIGURES 185–188 ; ppf) broad and elongate; maxillary palp slightly to much longer than head capsule, composed of minute palpomere I ( Fig. 185 View FIGURES 185–188 ; mxp1), slender, curved, distinctly but only slightly broadening distad palpomere II ( Figs 185, 187 View FIGURES 185–188 ; mxp2), palpomere III ( Fig. 187 View FIGURES 185–188 ; mxp3) strongly elongate, strongly and gradually broadened distad, with transverse or indistinctly obtuse distal margin, palpomere IV ( Fig. 187 View FIGURES 185–188 ; mxp4) about as long as III, broadening to distal third and with rounded or slightly subtriangular apex. Surface of palpomere IV, and sometimes also III, with sparsely distributed porous fields ( Fig. 188 View FIGURES 185–188 ; pf). Palpomeres III and IV slightly (sometimes indistinctly) flattened, II–IV covered with relatively long and dense setae. Labium ( Figs 177 View FIGURES 176–179 , 184, 186) with broad and short submentum ( Fig. 177 View FIGURES 176–179 ; smn) posteriorly not demarcated from gular region, densely setose and lacking an outstanding pair of anterior or subanterior lateral setae; mentum (Fig. 184; mn) subtrapezoidal and strongly transverse, with anterior margin very deeply emarginate, so that anterolateral corners form triangular and usually pointed lobes projecting anterad; prementum (Fig. 184; pm) long, subtrapezoidal, broadest distally, lacking demarcated ligula, with several pairs of submedian anterior setae, with broadly separated bases of labial palps; lateral hypopharyngeal lobes (Figs 184, 186; lhl) moderately large, with conspicuously sparse, thick mesal setae; hypopharynx ( Fig. 186 View FIGURES 185–188 ) with two lateral groups of long trichia; labial palp composed of three palpomeres: palpomere I (Fig. 184; lp1) small, elongate, strongly broadening distad, palpomere II (Figs 184, 186; lp2) largest, conspicuously enlarged, long and broad, approximately barrel-shaped, palpomere III (Figs 184, 186; lp3) very small and narrow in relation to III, as long as about half length of III and very narrow, pointed.
Prothorax ( Figs 155–156 View FIGURES 153–158 , 159–160 View FIGURES 159–162 , 189 View FIGURES 189–193 ) elongate, strongly convex but usually with flattened dorsum, broadest near anterior third. Pronotum with anterior and posterior margins arcuate (anterior margin sometimes nearly straight), sides rounded in anterior half and sinuate or (rarely) nearly straight in posterior half; anterior and posterior corners obtuse-angled or broadly rounded; pronotal base lacking pits and groove. Prosternum ( Fig. 189 View FIGURES 189–193 ) with basisternal part ( Fig. 189 View FIGURES 189–193 ; bstr) subequal in length to coxal part ( Fig. 189 View FIGURES 189–193 ; cxst). Prosternum laterally completely fused with hypomera. Coxal region anteriorly and laterally without marginal carina; postcoxal hypomeral lobes ( Fig. 189 View FIGURES 189–193 ; pchl) conspicuously large, rounded and strongly projecting anteromesad and overlapping with (but not fused to) posterolateral lobes of prosternum, so that procoxal cavities are not open, but entirely delimited posterioly by hypomeral lobes. Prosternal intercoxal process developed as a narrow and weakly elevated carina in intact beetles hidden between procoxae. Ventral surface of prothorax densely setose.
Mesoventrite ( Figs 191–192 View FIGURES 189–193 ) subtrapezoidal, broadening posteriorly. Prepecti ( Fig. 191 View FIGURES 189–193 ; pre) moderately long and together with anteromedian mesoventral area forming a relatively short 'collar', which is strongly impressed just behind its anterior ridge, anterior margin of impression with a short median subtriangular projection. Impressed area deep and broad, forming a well-defined setose impression ( Figs 191–192 View FIGURES 189–193 ; si) densely filled with long setae, with its posterior margin sharply demarcated from median precoxal portion of mesoventrite (but posterior margin usually obscured by dense setae). Mesoventral intercoxal process ( Figs 191–192 View FIGURES 189–193 ; msvp) reaching middle of mesocoxal cavities; short and very broad, subtrapezoidal, weakly convex, broadly separating mesocoxae, posteriorly separated from metaventrite by a transverse groove (often obscured by setae but discernible in transparent mounts ( Fig. 192 View FIGURES 189–193 )), posterior margin of mesoventral process nearly straight. Mesanepisterna relatively narrow and strongly elongate, demarcated from median part of mesoventrite by a distinct ridge and from mesepimera by complete suture; mesepimera elongate, indistinctly demarcated from metepimera, not exposed in ventral view.
Mesonotum with cordiform, broad mesoscutellum ( Fig. 190 View FIGURES 189–193 ; scl2) with pointed apex, in intact specimens not visible between elytral bases, or only its very tip discernible; scutoscutellar suture absent.
Metanotum ( Fig. 193 View FIGURES 189–193 ) partly reduced, with lightly sclerotized mesoscutum, but only slightly shortened alacristae ( Fig. 193 View FIGURES 189–193 ; ala); hind wings absent.
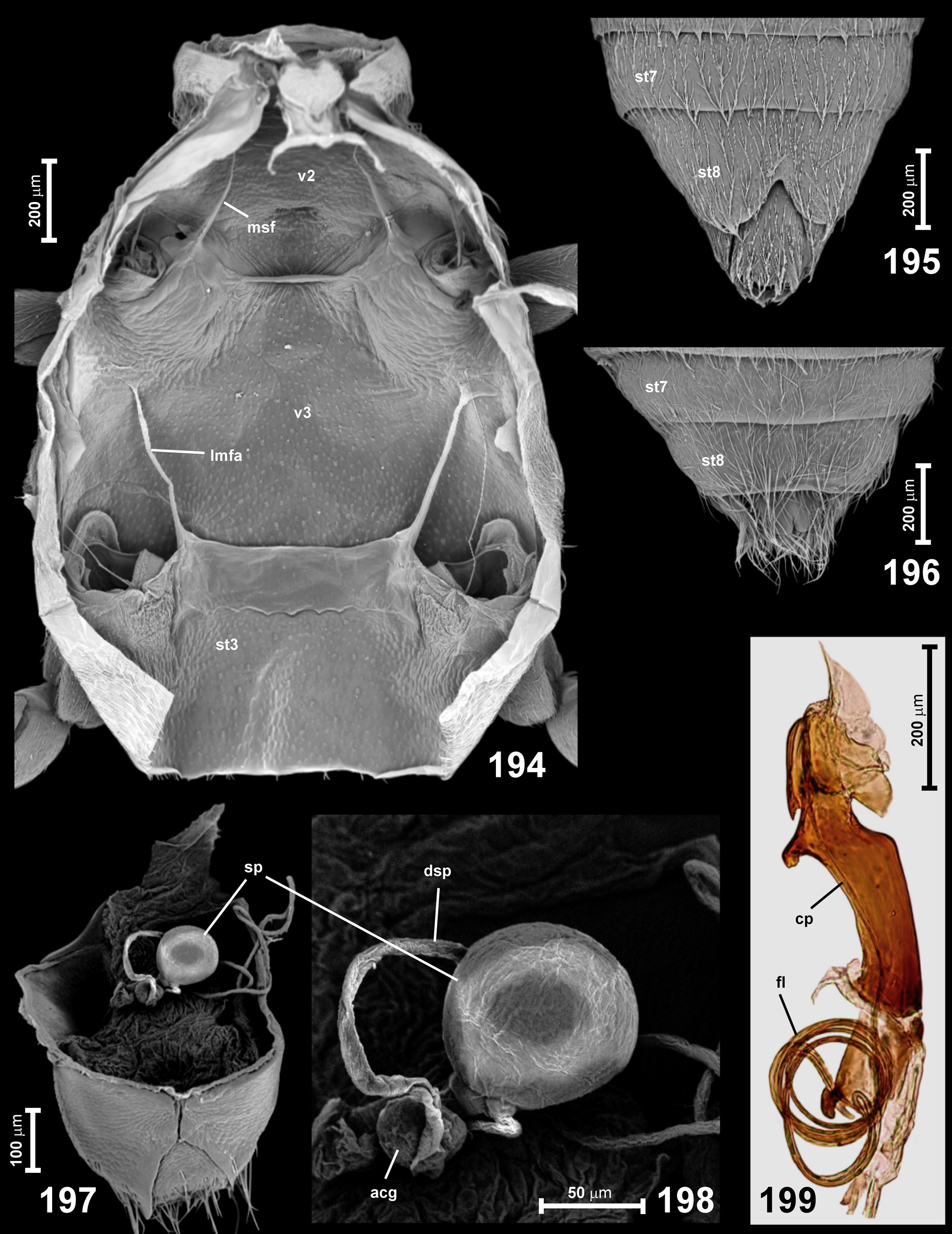
Metaventrite ( Figs 191 View FIGURES 189–193 , 194 View FIGURES 194–199 ) short, subrectangular and transverse, with lateral margins rounded; mesocoxal cavities with all margins non-carinate; posterior margin of metaventrite deeply bisinuate laterally (in front of each metacoxa) and with a broad metaventral intercoxal process ( Fig. 191 View FIGURES 189–193 ; mtvp) with shallowly concave or straight posterior margin; anteriorly metaventrite forming a short anterior metaventral process ( Figs 191–192 View FIGURES 189–193 ; amvp) similar in shape to mesoventral intercoxal process and meeting the latter at middle of mesocoxal cavities; metaventral foveae absent. External admetacoxal part of posterior metaventral margin lacking adcoxal carinae, but with adcoxal expansions ( Fig. 191 View FIGURES 189–193 ; ade). Metanepisterna relatively narrow, partly visible in ventral view, narrowing posteriorly; metepimera 2–3 times as broad as metanepisterna, with not demarcated inner and outer components, posteriorly extending far behind metacoxae.
Metendosternite (metafurca) with stem much broader than long and with broadly separated, divergent lateral furcal arms ( Fig. 194 View FIGURES 194–199 ; lmfa), lacking median longitudinal projection.
Legs ( Figs 155–156 View FIGURES 153–158 , 159–160 View FIGURES 159–162 , 191 View FIGURES 189–193 ) very long and slender. Pro- and mesocoxa short subconical, metacoxa with nearly hemispherical basal part and subconical distal part. Mesocoxa lacking coxal bristles. All trochanters short and subtriangular; femora weakly clavate; tibiae slender; tarsi long and slender, nearly subcylindrical, tarsomeres I–V reducing in length, tarsomere V strongly elongate, with curved and slender claws lacking elongate costae; empodial region was not studied.
Elytra ( Figs 155–156 View FIGURES 153–158 , 159–160 View FIGURES 159–162 ) oval, strongly convex, lacking humeral calli and basal impressions, with rounded apices; elytral disc with superficial, indistinct and incomplete longitudinal rows of fine punctures, often barely discernible; microsculpture fine. Elytra densely setose, setae short and nearly recumbent.
Abdomen ( Figs 191 View FIGURES 189–193 , 195–196 View FIGURES 194–199 ) with sternite III firmly fused with metaventrite; much longer than sternite IV, but shorter than IV–VI together; sternite VIII in male distinctly, often deeply emarginate ( Fig. 195 View FIGURES 194–199 ).
Aedeagus (illustrated in Bordoni & Castellini (1973), Jałoszyński et al. (2015, 2018), Lhoste (1936)) elongate, but not conspicuously long, with asymmetrical median lobe and asymmetrical parameres, one shorter than the other, both always well-developed; flagellum very long and forming several coils ( Fig. 199 View FIGURES 194–199 ; fl). Ejaculatory duct with elongate and narrow sperm pump lacking funnel-like structures. Aedeagus with elongate copulatory piece ( Fig. 199 View FIGURES 194–199 ; cp) with membranous endophallus permanently everted, and only inflated during copulation, with distal end of flagellum permanently fixed, so that flagellum is not extricable. Aedeagus in repose positioned asymmetrically inside abdomen, with basal orifice lateral or dorsolateral.
Spermatheca ( Figs 197, 198 View FIGURES 194–199 ; sp) subglobose, with relatively large accessory gland.
Sexual dimorphism distinct, females distinctly larger and with relatively broader elytra than males; males with emarginate, females with rounded abdominal sternite VIII; males often with slightly curved apices of protibiae.
Characteristics. Larvae. De Marzo (1983, 1984) described eggs, all larval instars and the pupa of the European Palaeostigus pilifer (Kraatz, 1879) ; Newton (1991) illustrated some structures for the Turkish Palaeostigus ruficornis schimitscheki (Machulka, 1944) ; and Grebennikov & Newton (2009) illustrated some larval structures of the South African Palaeostigus bifoveolatus ( Boheman, 1851) . Larvae ( Figs 200–201 View FIGURES 200–201 ) campodeiform, subparallel or with strongly narrowing abdomen, slightly flattened; head, tergal and sternal plates in living mature larvae dark brown to nearly black, remaining areas creamy white; young instars orange. Body sparsely covered with long unmodified setae and dense asperities forming patterns among smooth areas of tergal plates, additionally with sparse short leaf-like setae with elongate ribs; frontal impression with setae covered with irregular convexities. Head prognathous and slightly tilted ventrad, lacking 'neck', with one stemma at each side; epicranial stem and frontal sutures distinct but short, together with antennal insertions shifted to posterior half of head capsule; nasale with a row of several short setae with papillate insertions. Head with large glandular impression at the junction of epicranial stem and frontal sutures, impression surrounded by or filled with short modified setae (but first instars without impression). Antenna about twice as long as head or slightly shorter, antennomeres subcylindrical and not broadened distad, antennomeres I and II very long and similarly broad, antennomere II subdivided into three sections, antennomere III vestigial, developed as a barely discernible papilla adjacent to base of strongly elongate, slightly asymmetrical, subconical and pointed accessory appendage. Mandibles falciform, moderately slender, pointed, each with one submedian mesal tooth; stipital projection of maxilla divided into two very short and broad, densely setose lobes; maxillary palp longer than head, with palpomere I short and II and III strongly elongate; labial palp with palpomere I longer than II, both subconical. Thoracic tergites with ecdysial line visible as a smooth elongate median stripe among lateral fields of dense asperities. Abdomen with ten segments, all except X (or IX and X) transverse; segment X elongate; urogomphi absent. Sternal plates on thorax and abdomen reduced to small, paired (2–4) and setose sclerites. Legs conspicuously long and slender, with particularly densely setose tibiotarsi. Spiracles annular, lateral, nine pairs: one on mesothorax and eight pairs on abdominal segments I–VIII.
Composition and distribution. Palaeostigus comprises 11 species and 14 subspecies distributed in Asia ( Turkey), Europe ( Albania, Austria, Bosnia & Herzegovina, Croatia, France, Greece, Italy, Portugal, Slovenia, Spain), and in South Africa ( Fig. 166 View FIGURE 166 ).
Natural history. Most species (both Mediterranean and South African) live in large and relatively dense populations restricted to small areas. Adults are diurnal, they can be found near streams or rivers in warm places that provide some rocks, logs and vegetation for the adults to climb and moist leaf litter microhabitats where the larvae develop ( Figs 202–207 View FIGURES 202–207 ). In places inhabited by these beetles it is often possible to observe hundreds of individuals; adults walk on the ground, on mosses, twigs and logs, climb rocks and bushes. Frequently such places are relatively small and can be restricted to a 20-m stripe of ground along a stream, outside of which beetles cannot be found (Jałoszyński, pers. obs.).
In Europe, females of Palaeostigus lay eggs in autumn and the larvae develop during winter (De Marzo 1983); the last-instar larvae can still be found in the beginning of April (Jałoszyński, pers. obs.). Later during summer, at least in Europe, places where beetles live frequently dry and beetles disappear; it is unclear whether they die, enter a diapause, or migrate to unknown shelters. Pérez Fernández et al. (2013) reported a common occurrence of the Iberian P. palpalis (Letreille, 1804) at mouths of caves in the Sistema de la Murcielaguina and even used this species as an indicator to locate new entrances to this cave system. It is possible that during the hot and dry season beetles enter caves or underground microhabitats.
Adults of Palaeostigus kept for a long time in captivity and offered a broad spectrum of potential prey, readily attacked and devoured small caterpillars (tentatively identified as Lecithoceridae ) that were obtained by sifting leaf litter from which also numerous beetles were collected ( Figs 208–213 View FIGURES 208–213 ). During attack, beetles were not using the antennal spines to catch their prey. Once a caterpillar was detected by palpating with tips of antennae and then with maxillary palps, the antennae were spread so that during the attack and during nearly entire feeding the spines on scape and pedicel did not touch the prey. Beetles used their mandibles to catch the caterpillar in any place on its body, and they ate captured prey while it was still alive. When the attack started from the tip of the abdomen, as in Fig. 208 View FIGURES 208–213 , prey's movements ceased only when about half or even more of its body was consumed. Beetles continuously moved their mandibles to damage prey's cuticle and increase the surface for digestive juices. The feeding process documented in Figs 208–213 View FIGURES 208–213 took 1 hour and seven minutes; the abandoned remains were hardly recognizable as a lepidopterous larva. When given large, dead caterpillars of noctuids or geometrids with their cuticle pierced or cut, so that some body fluids spilled outside, beetles quickly detected this source of food and gathered around to feed on the liquids ( Figs 214–216 View FIGURES 214–216 ). Live and undamaged large caterpillars were not attacked. During about two months of laboratory observations, adults of P. palpalis did not catch any living springtails; beetles showed some interest toward any moving objects, including Collembola, but were not able to get close enough to any springtails for a successful attack. The touching with antennae caused springtails to jump away and avoid being captured. Only when dead or half-dead (pierced with a needle; still alive, but not able to jump or walk) tomocerid springtails were found by beetles, they were readily eaten ( Fig. 217 View FIGURES 217–220 ). Beetles also fed on living enchytraeids found between leaves or on the surface of soil in their containers ( Fig. 218 View FIGURES 217–220 ), attacking them and eating in a similar way as small caterpillars. Beetles also readily scavenged on a variety of dead arthropods, including large freshly killed flies ( Fig. 219 View FIGURES 217–220 ); they also used as food any raw or processed meat provided, including pieces of ham or mortadella ( Fig. 220 View FIGURES 217–220 ). They gathered around large pieces of solid food and used their mandibles to chew on it.
It was previously hypothesized that long spines on the scape and pedicel of Mastigini may be used as a 'springtail-trap', a catching device that may work in a similar way as spiny antennae of springtail-eating carabid Loricera Latreille (Jałoszyński 2016a; Yin et al. 2017a). These hypotheses were based solely on the antennal structure and required verification. Field observations and laboratory experiments (not only with Palaeostigus , but also with Stenomastigus , which has a similar antennal structure) carried out during the present project in South Africa and Spain, did not provide any evidence to support this view. In a contrary, not even once the beetles were observed using their antennal spines to catch (or to try to catch) any prey organisms, and during attack and feeding on various arthropods beetles kept their antennae spread in such a way that the spines did not touch the prey. Interestingly, Yin et al. (2017a) published their study on Mastigini fossils focused on the catching antennal apparatus knowing about my results clearly falsifying the initial 'springtail trap' hypothesis (correspondence with Z.-W.Yin).
Function of the enlarged and spiny scape and pedicel in Palaeostigus and other Mastigini remains unclear. The large groups of porous fields between the rows of spines suggest a sensory function, but it does not explain the spines, which in fact would interfere with moving the ventral surface of scape and pedicel close to any objects. The only occasions when the spines possibly directly touch objects in front of beetles are frequent encounters between individuals of the same species, under laboratory conditions forced by keeping many individuals on a relatively small surface, but observed to occur also in nature, as Palaeostigus lives in large groups and beetles continuously walk around and often meet each other. When two beetles meet face to face, they engage in a rapid palpating each other with antennae; it lasts only a second or two, then beetles stop palpating each other and each walks away. The action of antennae is very quick; beetles resemble two ant workers meeting, but the interaction is very quickly terminated. It seems that the antennal spines of beetles engaged in such a brief interaction touch, but movements are too fast to observe any details (my attempts to photograph such meetings failed).
Beetles walking on a ground, gathering around dead arthropods or pieces of meet, and 'communicating' among themselves appear similar to ants. The body form, geniculate antennae and the short and dense vestiture of setae that appears silverish on black European adults of Palaeostigus enhance this impression, as Formica ants that are similar in size, shape, pigmentation and behavior can be commonly found in places where Palaeostigus lives (Jałoszyński, pers. obs.). It is possible that beetles benefit from their apparent 'myrmecomorphy', as it was demonstrated that some predators avoid not only ants but also ant-like prey (e.g. Taniguchi et al. (2005)).
Copulation in South African Palaeostigus was observed (Jałoszyński et al. 2015); males have relatively short aedeagi, and they mount females with both individuals facing the same direction, with the tip of copulatory piece inserted into the female's gonopore, and parameres touching female's terminal abdominal segments; because of the different length of parameres, the aedeagus is twisted and positioned asymmetrically.
Remarks. Palaeostigus and Mastigus are morphologically very similar; see Remarks for the latter genus. All nominal species were treated by Bordoni & Castellini (1973) and Leleup (1968).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scydmaeninae |
|
SuperTribe |
Mastigitae |
|
Tribe |
Mastigini |