Underwoodisaurus seorsus, Doughty, Paul & Oliver, Paul M., 2011
|
publication ID |
https://doi.org/10.5281/zenodo.204380 |
|
DOI |
https://doi.org/10.5281/zenodo.5673811 |
|
persistent identifier |
https://treatment.plazi.org/id/22580E10-6C32-4E3E-A8DD-F998FA8CE428 |
|
treatment provided by |
Plazi |
|
scientific name |
Underwoodisaurus seorsus |
| status |
sp. nov. |
Underwoodisaurus seorsus sp. nov.
Pilbara Barking Gecko Figs. 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5
Holotype. WAM R157525, adult female with original tail, Packsaddle Range ( 22.9144ºS, 118.9158ºE, elevation 638 m), Pilbara region, Western Australia, collected by P. Cullen and M. Menz on 7 May 2004.
Paratypes. WAM R129895 (male), West Angelas, 100 km north-east of Newman ( 23.1833ºS, 118.8142ºE, elevation 775 m) on 14 June 1997; WAM R157520 (male), WAM R157522 (female), WAM R157513 (juvenile), Packsaddle Range ( 22.9144ºS, 118.9158ºE, elevation 861 m) collected on 7 May 2004; WAM R163638, female, 60 km north of Tom Price ( 22.1191ºS, 117.9183ºE, elevation 579 m) collected on 18 November 2008; all from the Pilbara region, Western Australia.
Diagnosis. A typically-sized (to ~ 100 mm SVL) Underwoodisaurus with transverse subdigital lamellae, minute anterior loreals compared to larger posterior loreals, labial scales larger than neighboring scales, unreduced phalangeal formula (2.3.4.5.3/2.3.4.5.4), and long original tail gradually tapering to tip. Distinguished from U. milii by possessing an elongate snout (NE/IN: 1.74–1.89), relatively long limbs (ArmL/SVL: 0.18–0.20; LegL/SVL: 0.20–0.22) and digits (4FL/SVL: 0.064–0.084; 4TL/SVL: 0.076–0.095), higher density of smaller enlarged tubercles scattered across the dorsum but with lower, more acute tubercles with anterior keel more common and conspicuous, relatively deep mental (projecting to level of, or beyond, first secondary infralabial) and often terminating posteriorly in a point ( v. rounded ), enlarged tubercles on original tails not forming transverse rows. Reddish-brown ground color with relatively plain head without light blotching or patterning, dorsal pattern consisting of sparsely scattered small pale tubercles and a narrow band across the nape.
Description. A large carphodactylid gecko with large head, long slender limbs and digits, and long tail terminating in a point ( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 ). Table 2 presents measurements and scores for meristic characters. Large and triangular head, nearly as wide as body, slightly depressed, terminating in a relatively straight-edged blunt snout; large and protruding eyes with overhanging supraciliary ridge, medial portion of dorsal eye bulges separated by 12–15 scales; dorsal skin on head not loose; wide nostrils in lateral view, narrow dorsally, directed posteriorly; vertical and elongate ear opening, recessed tympanum; labial scales enlarged relative to neighboring scales, first largest, then gradually decreasing along jaw; wide rostral, lacking a medial crease, and not in contact with nostril; anterior edge of nostril bordered anteriorly by 3 enlarged scales and 8–10 fine postnasal scales; relatively straight, slightly concave loreal region; extremely small loreal scales closest to nostrils compared to larger loreal scales nearer to the eye and elsewhere on head; mental deep, usually extending beyond scale posterior to first secondary infralabial, and ending in a sharp point; narrow neck, approximately half the width of head.
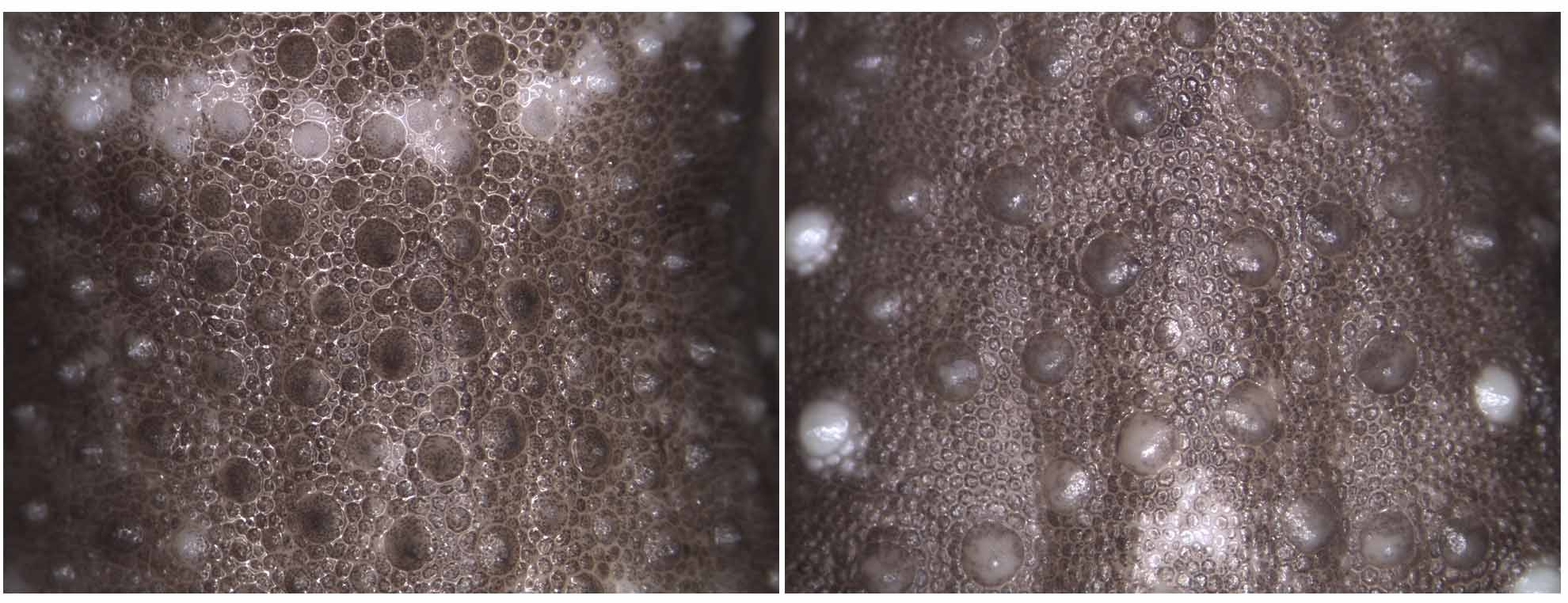
Body covered with small relatively flat scales with much larger tubercles scattered across dorsum with higher density towards flanks ( Fig. 5 View FIGURE 5 ); tubercles heterogeneous in size; tubercles round or slightly keeled anteriorly (especially in nuchal region), apex directed posteriorly; ventral surface covered with flat slightly oblong scales, scales on venter larger than those on dorsum; scales below neck and gular region small; scale rows medial to infralabials enlarged relative to gulars and in ca. 6 rows.
Very long and slender limbs, pentadactyl; long fingers (4FL/SVL: 0.064–0.084) and toes (4TL/SVL: 0.076– 0.095), terminating in a sharp claw; limbs covered in fine scales with scattered moderately-sized tubercles; digits moderately compressed and covered in small scales on the dorsal and lateral surfaces, but with narrow lamellae that span the width of the fingers (4FLam: 15–18) or toes (4TLam: 18–23); no expanded lamellae at tips; claw surrounded by sheath formed by a ring of enlarged scales.
Tail long (78 and 95% of SVL), constricted at base widening ca. 5 mm past cloaca in two adult specimens with original tails; proximal half wide with thick medial portion and tapering laterally; scattered dorsal tubercles on proximal half, tending not to form transverse rows, and encircled by a row of scales at base of tubercle; distal half of tail narrows, gradually tapering to a fine point. No pre-anal or femoral pores; 11 or 13 enlarged cloacal spurs to either side of base of tail of single adult male specimen (WAM R157520), 4–6 enlarged cloacal spurs on females; hemipenes strongly bifid.
Coloration. Ground color light to medium reddish-brown, overlain with numerous small pale spots corresponding to tubercles that tend to form irregular bands across the dorsum; plain and unpigmented ventral surface of body, legs, feet and tail; dark reddish brown original tail with five pale stripes: two on wide proximal half of tail consisting of separate small white spots and three on thin distal half of tail that are more defined but still broken ( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 ).
Variation. Meristic and mensural variation is provided in Table 2. The largest specimens were females, approaching 100 mm SVL. Pattern variation was slight, although the background coloration appeared darker in smaller individuals ( Fig. 4 View FIGURE 4 ). Regrown tails were more mottled, without the regular bands of original tails.
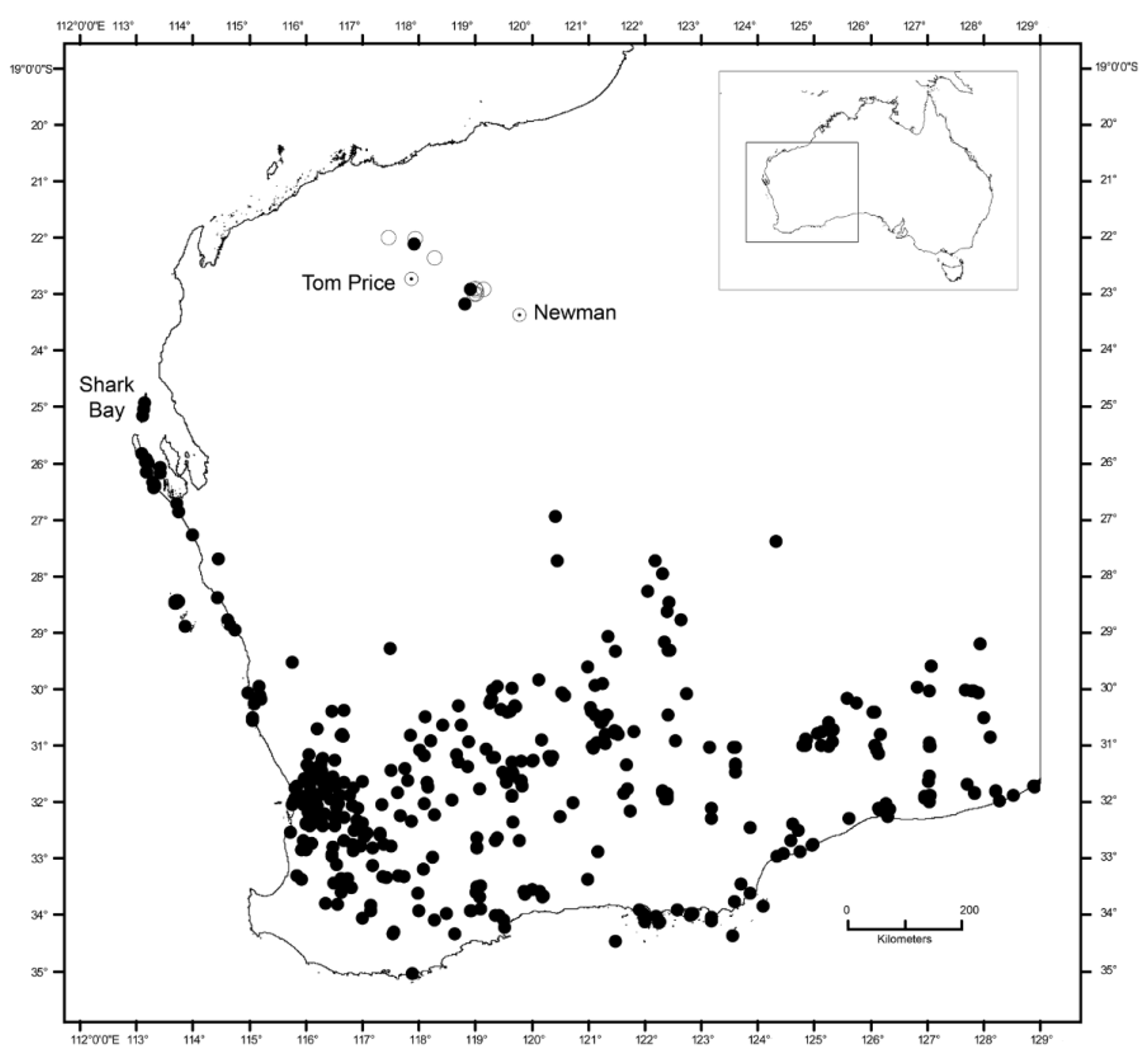
Distribution. Known only from the Hamersley Range in the Pilbara region of Western Australia: from north of Tom Price in the western Hamersley to West Angelas mine near Newman to the south-east ( Fig. 1 View FIGURE 1 ). These three points are supplemented by other observations of U. seorsus sp. nov. where specimens were not collected, but confirmed by observations of knowledgeable herpetologists and/or photographs examined by the authors (see Appendix 2; open circles in Fig. 1 View FIGURE 1 ). The closest populations of U. milii are approximately 450 km to the south-east near Wiluna and 600 km to the south-west in Shark Bay and offshore islands ( Fig. 1 View FIGURE 1 ).
Habitat. Encountered in rocky areas of the Hamersley Range. Some of the specimens from the type series were collected on a graded road running through a ‘ major gully’, and one was sheltering under a rock slab ( Menz & Cullen 2006). They have also been observed at the bottom of a rocky gorge with a low tree cover ( Thompson et al. 2009). Vegetation associated with other observations (Appendix 2) is shown in Fig. 6 View FIGURE 6 and consists of low sparse trees of Eucalyptus leucophloia , low shrubs of Acacia pilbara and Triodia wiseana (M. O’Connell & B. Maryan, pers. comm.).
Etymology. seorsus is Latin for ‘apart’ or ‘separate’ in reference to the large distance between the distributions of U. seorsus sp. nov. and U. milii . Used as a noun in apposition.
Comparison with other species. Underwoodisaurus seorsus sp. nov. can be distinguished from the two Nephrurus species in the Pilbara by absence of the enlarged knob at the end of the tail. In the absence of an original tail, N. wheeleri cinctus possesses large tubercles scattered on the dorsum and also enlarged scales on the head; in addition, this species has conspicuous bands. Nephrurus levis pilbarensis has multiple wide nuchal bands, whereas U. seorsus sp. nov. has only a single narrow band.
Although widely allopatric, Underwoodisaurus seorsus sp. nov. is only likely to be confused with U. milii . In overall appearance, U. seorsus sp. nov. is relatively more slender than U. milii , and has a longer snout, narrower head, more infralabials, longer limbs and toes ( Table 3 View TABLE 3 ), more numerous but smaller, more acute, and lower scattered dorsal tubercles, and a deeper angular mental scale. The pattern and color also differ: U. seorsus sp. nov. has a relatively simple dorsal pattern on both head and body comprised of widely scattered pale tubercles. In contrast, U. milii often has relatively well-defined transverse bands made up of larger spots, and usually has considerable areas of lighter patches on the head, including the labial scales and often extending across the top of the head ( Figs. 2 View FIGURE 2 , 4 View FIGURE 4 ). The pale-headed pattern is most apparent in populations from Shark Bay, one of the closest populations to U. seorsus sp. nov. and the type locality for U. milii ( Shea 2002) .
Remarks. While early biogeographic studies did not identify the Pilbara as an area of high faunal endemism ( Schall & Pianka 1978; Cracraft 1991), it is becoming increasingly clear that the Pilbara is a center of faunal endemism within Australia ( How & Cowan 2006; Powney et al. 2010; Doughty et al. 2011a, submitted). Based on the limited numbers of studies that have been published it appears that endemic Pilbara lineages have a wide range of geographic associations; some are southern isolates of northern lineages (Fitch et al. 2006; Catullo et al. 2011), some are sister to arid zone lineages ( Aplin et al. 2006; Pepper et al. 2006) and some are northern isolates of lineages with otherwise more southerly distribution ( Doughty et al. 2008). This diverse pattern of relationships of Pilbara taxa to other regions is not surprising given the size, geographic complexity and age of the Pilbara landform.
Within the Pilbara , a disproportionate number of endemic species have saxicoline habits compared to other parts of the arid zone ( Doughty et al. 2011a, submitted). The occurrence of U. seorsus sp. nov. in the Pilbara is consistent with the pattern of saxicoline endemics, as individuals have only been collected from rocky areas, and U. milii from their extensive southern range are frequently associated with rocks (e.g. Storr et al. 1990; Swan et al. 2004). The distribution of U. seorsus sp. nov. and U. milii suggests that their common ancestor was once more widely distributed, but that aridification and/or increasing temperature eliminated populations in the Gascoyne region. The rocky gorges and moderately high elevations of the southern Pilbara ranges may have acted as a relatively moist and potentially cooler refugium, allowing an isolated population of Underwoodisaurus to persist especially the rocky gorges at moderately high elevations where individuals have been observed to occur. Relative to southern U. milii , U. seorsus sp. nov. are much more slender with a longer snout, larger scales and longer digits and limbs. This elongation of the body and limbs may aid climbing in the rocky areas this species appears to favor, but further observations of behavior are necessary to test this idea.
Within the Pilbara , U. seorsus sp. nov. seems to be rare and have a relatively small distribution. Despite extensive survey effort in recent years they have only been found at a small number of sites. This combination of rarity and small relictual distribution indicates this species may be of conservation concern. The possible effects of increases in global temperature in the coming decades on what is seemingly already a relictual species are of particular concern. We recommend that U. seorsus sp. nov. is classified as Priority 2 (Department of Environment & Conservation, Western Australia) which will afford this species a high level of protection within the state.
TABLE 3. Comparison of average and range of key ratios and meristic characters for Underwoodisaurus seorsus sp. nov. and U. milii. Ratios are based on adult specimens of both species, while meristic counts (*) include the entire type series of U. seorsus sp. nov.
| U. seorsus sp. nov. N = 4 or 6* | U. milii N = 20 | Statistics: Mann-Whitney U | |
|---|---|---|---|
| SVL | 88.3 (78.0–99.0) | 84.7 (72.0–100.0) | 47.5, P = 0.627 |
| TrunkL/SVL | 0.47 (0.45–0.48) | 0.44 (0.39–0.47) | 78.0, P = 0.001 |
| ArmL/SVL | 0.19 (0.18–0.20) | 0.17 (0.16–0.18) | 77.0, P = 0.001 |
| LegL/SVL | 0.21 (0.20–0.22) | 0.20 (0.17–0.21) | 69.0, P = 0.023 |
| HeadL/SVL | 0.28 (0.27–0.28) | 0.28 (0.26–0.29) | 44.0, P = 0.794 |
| HeadW/SVL | 0.20 (0.19–0.21) | 0.21 (0.19–0.22) | 69.0, P = 0.023 |
| HeadD/HL | 0.72 (0.68–0.73) | 0.75 (0.69–0.82) | 50.0, P = 0.477 |
| NE/IN | 1.83 (1.74–1.89) | 1.64 (1.44–1.81) | 78.5, P <0.001 |
| SupLab* | 13.2 (12–14) | 12.4 (10–14) | 83.0, P = 0.176 |
| InfLab* | 12.0 (11–14) | 10.9 (9–12) | 95.0, P = 0.033 |
| 4FL/SVL | 0.071 (0.064–0.084) | 0.061 (0.052–0.070) | 58.0, P = 0.183 |
| 4TL/SVL | 0.084 (0.076–0.095) | 0.074 (0.063–0.085) | 67.0, P = 0.037 |
| 4Flam* | 16.7 (15–18) | 16.1 (14–19) | 80.0, P = 0.242 |
| 4TLam* | 19.8 (10–23) | 19.3 (17–23) | 71.5, P = 0.533 |
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Gekkota |
|
Family |
|
|
Genus |