Rhagoletis Bushi
|
publication ID |
https://doi.org/10.1093/isd/ixy016 |
|
publication LSID |
lsid:zoobank.org:pub:5C5EAC90-1213-45B3-A985-D35C525EC210 |
|
persistent identifier |
https://treatment.plazi.org/id/232ABD59-FF8C-FFCD-FC84-48FFFD9B3B55 |
|
treatment provided by |
Felipe |
|
scientific name |
Rhagoletis Bushi |
| status |
|
Rhagoletis Bushi View in CoL View at ENA : A New Species
The description of R. bushi represents a new North American Rhagoletis species. We present three lines of evidence to support the new species. First, R. bushi has unique and diagnostic morphological characters, second R. bushi infests the fruits of a unique host plant for Rhagoletis , and third R. bushi is genetically distinct from other species in the genus.
Morphological characters can be used to diagnose R. bushi . A combination of wing patterning, head patterning, wing cell shape, and pleural setae characters was not described by Jenkins (1996), which are useful for readily identifying R. bushi without resorting to genitalic characters (but see Diagnosis). Although R. bushi may be mistaken for R. ribicola (Doane) , R. berberis (Curran) , or R. juniperia in the absence of genitalic characters or host plant data.
The only known Rhagoletis species to infest S. argentea (buffaloberry) is R. bushi . The use of a particular host plant can be a proxy for species identification in Rhagoletis , although there are important but rare exceptions where host-specific flies have been reared from ‘non-natal’ hosts that likely do not represent established populations ( Bush 1966, Yee and Goughnour 2008, Hood et al. 2012a, Yee et al. 2015). There are two other species in the genus Shepherdia : S. canadensis (L.) Nutt. (Canadian buffaloberry) and S. rotundifolia Parry (roundleaf buffaloberry), but it is unknown whether R. bushi or other Rhagoletis infest these species. Rhagoletis bushi is unlikely to infest S. rotundifolia as its range ( Arizona and Utah) does not overlap with that of S. argentea and grows in a warmer, drier climate. The range of S. canadensis does overlap with S. argentea and the two species fruit at similar times (mid to late summer; Soper and Heimburger 1982); thus, S. canadensis may represent a viable host. However, Bush attempted to rear Rhagoletis from S. canadensis and did not find them infested (unpublished data). There are few published records of attempts to collect Rhagoletis from Shepherdia spp., and those that exist have either found S. argentea infested with
R. bushi ( Smith and Bush 1997) View in CoL or with nothing ( Glasgow 1933). There are few records of insects infesting the fruit of S. argentea View in CoL , but buffaloberry is also a reported host plant of Drosophila suzukii (Matsumura) View in CoL and, possibly, other Drosophila ( Agbaba 2017) View in CoL .
R. bushi View in CoL is genetically distinct from other Rhagoletis View in CoL . All of the gene sequences we used were able to discriminate R. bushi View in CoL from other members of the genus. In phylogenetic analysis, we found all loci to be reciprocally monophyletic with respect to R. bushi View in CoL individuals. Perhaps most useful for future genetic identification, the barcode region of COI is able to easily identify R. bushi View in CoL . Similarly, the insertion in the 28S gene in R. bushi View in CoL may also serve as a useful diagnostic genetic character. Otherwise, we found a total of 17 autapomorphic positions for R. bushi View in CoL across all gene alignments (three in COI, four in CAD, five in period, one in AATS, and four in 28S; Supp Table 4 [online only]). The average sequence divergence (%) across all genes between R. bushi View in CoL and R. electromorpha View in CoL , R. persimilis View in CoL , R. tabellaria View in CoL , and R. pomonella View in CoL was 1.46, 1.48, 1.43, and 2.89, respectively.
Phylogenetics and Evolution of the tabellaria
Species Group
The tabellaria View in CoL species group is itself monophyletic and appears to be sister to the pomonella View in CoL species group.The existence of Rhagoletis View in CoL species groups has been reported by Bush (1966) and subsequent investigations have supported their existence ( McPheron and Han 1997, Smith and Bush 1997, Smith et al. 2005). Hamerlinck et al. (2016) first reported evidence of a sister relationship between the tabellaria View in CoL and pomonella View in CoL groups based on DNA sequences of alleles at three loci (28S, CAD, and COI), and this relationship has been further supported by the additional sequences presented here. Phylogenetic relationships within the genus are relatively unresolved beyond the tabellaria View in CoL and pomonella View in CoL groups. Within the North American species groups, there appears to be a close relationship between the cingulata View in CoL , suavis View in CoL , and ribicola View in CoL groups ( Hamerlinck et al. 2016).
We hypothesize a sister relationship between R. tabellaria View in CoL and R. electromorpha View in CoL and present evidence to support this based on nucleotide sequences and morphological characteristics. Based on the Bayesian phylogenetic analysis performed on concatenated gene sequences, we inferred a sister relationship between R. electromorpha View in CoL and R. tabellaria View in CoL , however, with only moderate confidence (BPP = 0.76; Fig. 4 View Fig ). The ML analysis did not resolve tabellaria View in CoL group species-level relationships. Nucleotide sequences are relatively phylogenetically inconclusive in the absence of other data. Future research plans for the tabellaria View in CoL group include the use of next-generation sequencing techniques to generate draft genomes to resolve phylogenetic relationships and address other systematic questions.
Morphological data further support a sister relationship for R. tabellaria and R. electromorpha . Of the characters analyzed by Jenkins (1996) that are relevant to the tabellaria group, all the (male) genitalic characters support a R. tabellaria–R. electromorpha grouping. Specifically, both species have an aedeagus with a gland-like tubular sac (basiphallic vesica), which appears to be a derived character, shared by no other known Rhagoletis species. Another derived character shared by R. tabellaria and R. electromorpha is the presence of only two spermathecae ( R. persimilis and R. bushi both have three) while possessing three spermathecal ducts. The spermathecal arrangement in R. tabellaria and R. electromorpha is consistent with the hypothesis that the common ancestor of the two species lost a spermatheca but not the associated duct. It is worth noting that R. ebbettsi , which we did not include in our analysis because of lack of material, has the same pattern of spermathecae and spermathecal ducts as R. tabellaria and R. electromorpha ( Bush 1966, Berlocher
1984). Collection and analysis of new R. ebbettsi individuals is necessary to have confidence in the species’ phylogenetic placement. Looking deeper, one of the defining characteristics of the pomonella group is the presence of three spermathecae (organized in a pair of unevenly sized spermathecae and a separated single spermatheca). The pomonella group arrangement of spermathecae is the same as in R. bushi and R. persimilis giving further evidence in support of the hypothesis that the loss of a single spermatheca in R. tabellaria and R. electromorpha is a derived character of sister species.
There is one (nongenitalic) morphological character relevant to the tabellaria group that does not support a R. tabellaria : R. electromorpha sister relationship: R. electromorpha has lateral scapular seta the same color as principle thoracic setae, while they are different in R. tabellaria ( Table 2; Jenkins 1996). However, we hypothesize that this character is homoplasious. Characters associated with male insect genitalia are under sexual selection ( Eberhard 1985, 2001, 2004; Huber and Eberhard 1997; Arnqvist 1998; Córdoba‐ Aguilar 2005; House and Simmons 2005; Arnqvist and Danielsson 2017) and evolve quickly relative to nongenitalic characters ( Arnqvist 1997, Hosken and Stockley 2004, Méndez and Córdoba-Aguilar 2004) meaning these characters may have a better phylogenetic signal than nongenitalic characters for recently diverged taxa ( Song and Bucheli 2010) such as those in the tabellaria group.
While the loci we sequenced allowed us to easily diagnose members of the tabellaria group, they were insufficient to resolve specieslevel relationships within the group. We found a panel of molecular autapomorphies for each species in the tabellaria group that may be useful for diagnostic applications (Supp Table 4 [online only]). Using DNA sequences for diagnosis is a useful alternative for members of the tabellaria group which can be potentially difficult to distinguish from each other on the basis of morphological data alone. However, more extensive sampling is needed to assess intraspecific variation and hidden diversity. Of the taxa we sampled, only R. tabellaria was sampled across its geographical range. The ranges of the other tabellaria species group members remain relatively unknown, although the ranges of their respective host plants are well characterized. For example, R. electromorpha is known to infest three species of dogwoods: C. drummondii , C. racemose , and C. foemina ( stricta) in the Eastern United States ( Berlocher 1984, Smith and Bush 1997), and diversity between populations infesting different species has not been characterized.
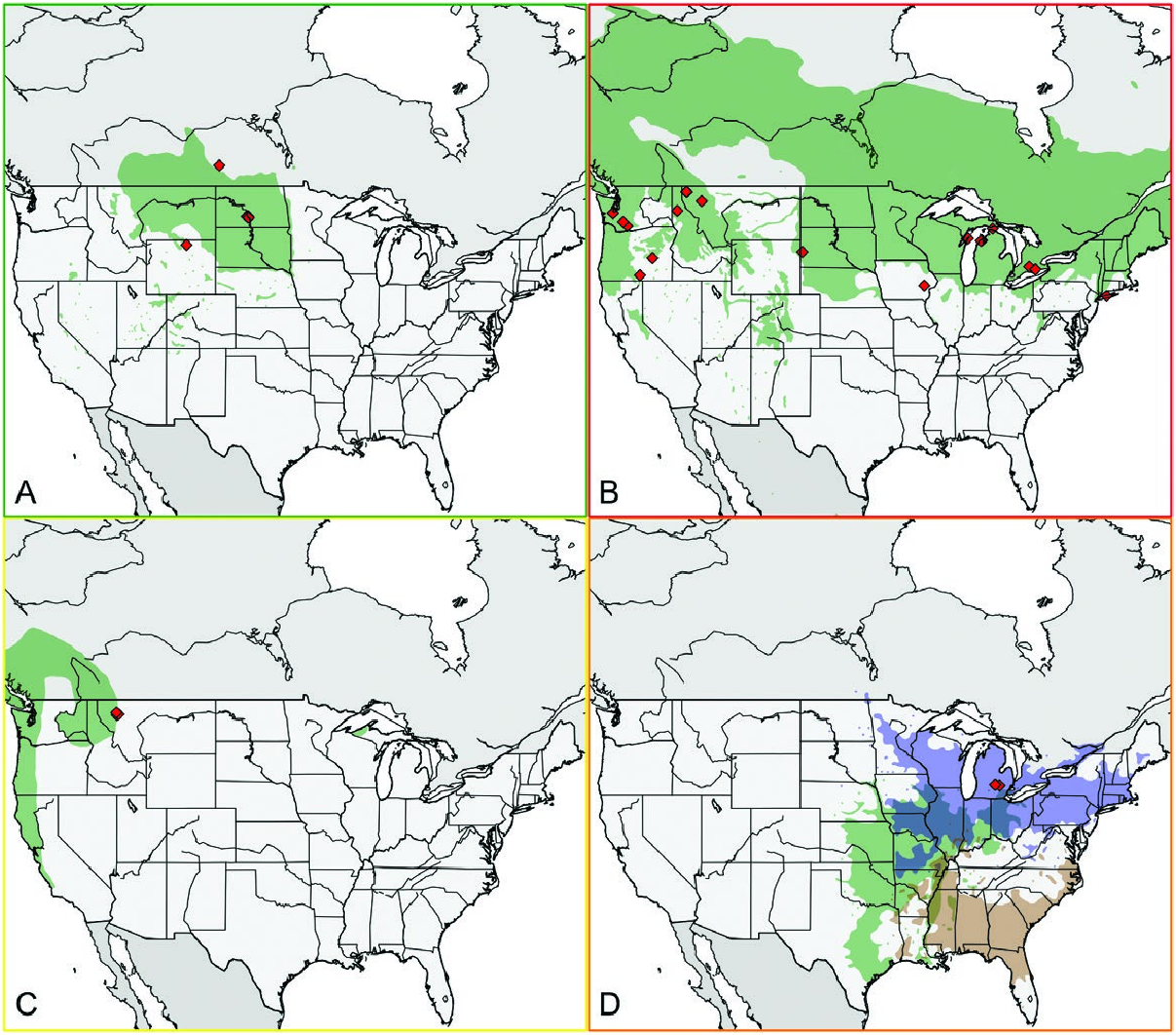
Hybridization between species in the pomonella group is known to occur and may also happen in the tabellaria group. The true phylogenetic relationship between species in the tabellaria group may be obscured in the presence of hybridization. In the pomonella group, R. pomonella , R. mendax , and R. zephyria are all known to hybridize with each other ( Bush 1966, Feder et al. 1999, Schwarz et al. 2005). Hybridization may have detrimental effects on subsequent generations, but it may also allow beneficial alleles to enter a population as evidenced by the introgression of alleles from R. zephyria to R. pomonella in western United States populations possibly conferring increased desiccation resistance on the latter ( Arcella et al. 2015). Every species in the tabellaria group has naturally occurring zones of sympatry with at least on other member ( Fig. 1 View Fig ), and Cornus is the host plant genus for both R. tabellaria and R. electromorpha potentially creating favorable conditions for hybridization.
Dogwoods ( Cornus ) may represent the ancestral host plant genus of the tabellaria species group and the pomonella group. There are two described species in the tabellaria group ( R. tabellaria and R. electromorpha ) and one in the pomonella group ( R. cornivora ) that infest dogwoods. The host dogwoods of the tabellaria and pomonella species are all in the subgenus Kraniopsis , and the hosts of R. tabellaria ( C. stolonifera ) and R. cornivora ( C. amomum ) are likely more closely related to each other than to the hosts of R. electromorpha ( C. foemina , C. drummondii , and C. racemose ; Xiang et al. 1996, 2006). The flowering dogwood fly is an undescribed pomonella group species, which attacks Cornus florida L., but we hypothesize that this represents an independent shift to a dogwood host because of the distant phylogenetic relationship between C. florida and the other dogwoods infested by Rhagoletis ( Xiang et al. 1996, 2006). Dogwoods have a broad distribution throughout the northern hemisphere which overlaps with all members of the tabellaria and pomonella species groups and would likely have been accessible to ancestral Rhagoletis populations.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhagoletis Bushi
| Hulbert, D., Jackson, M. D., Hood, Glen R. & Smith, J. J. 2018 |
R. electromorpha
| Berlocher 1984 |
R. electromorpha
| Berlocher 1984 |
R. electromorpha
| Berlocher 1984 |
R. persimilis
| Bush 1966 |